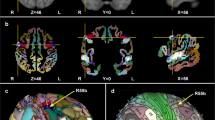
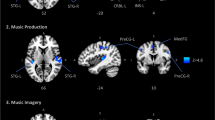
Abstract
Music consists of precisely patterned sequences of both movement and sound that engage the mind in a multitude of experiences. We move in response to music and we move in order to make music. Because of the intimate coupling between perception and action, music provides a panoramic window through which we can examine the neural organization of complex behaviors that are at the core of human nature. Although the cognitive neuroscience of music is still in its infancy, a considerable behavioral and neuroimaging literature has amassed that pertains to neural mechanisms that underlie musical experience. Here we review neuroimaging studies of explicit sequence learning and temporal production—findings that ultimately lay the groundwork for understanding how more complex musical sequences are represented and produced by the brain. These studies are also brought into an existing framework concerning the interaction of attention and time-keeping mechanisms in perceiving complex patterns of information that are distributed in time, such as those that occur in music.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Fuster, J.M. The prefrontal cortex - an update: time is of the essence. Neuron 30, 319–333 (2001).
Rizzolatti, G. & Luppino, G. The cortical motor system. Neuron 31, 889–901 (2001).
Ivry, R.B. The representation of temporal information in perception and motor control. Curr. Opin. Neurobiol. 6, 851–857 (1996).
Ivry, R.B. & Richardson, T.C. Temporal control and coordination: the multiple timer model. Brain Cogn. 48, 117–132 (2002).
Schöner, G. Timing, clocks, and dynamical systems. Brain Cogn. 48, 31–51 (2002).
Krampe, R.T., Engbert, R. & Kliegl, R. Representational models and nonlinear dynamics: irreconcilable approaches to human movement timing and coordination or two sides of the same coin? Brain Cogn. 48, 1–6 (2002).
Essens, P.J. & Povel, D.J. Metrical and nonmetrical representations of temporal patterns. Percept. Psychophys. 37, 1–7 (1985).
Povel, D.J. & Essens, P. Perception of temporal patterns. Music Percept. 2, 411–440 (1985).
Large, E.W. On synchronizing movements to music. Hum. Mov. Sci. 19, 527–566 (2000).
Large, E.W. & Palmer, C. Perceiving temporal regularity in music. Cognit. Sci. 26, 1–37 (2002).
Repp, B.H. Phase correction, phase resetting, and phase shifts after subliminal timing perturbations in sensorimotor synchronization. J. Exp. Psychol. Hum. Percept. Perform. 27, 600–621 (2001).
Repp, B.H. Detecting deviations from metronomic timing in music: effects of perceptual structure on the mental timekeeper. Percept. Psychophys. 61, 529–548 (1999).
Repp, B.H. Phase correction following a perturbation in sensorimotor synchronization depends on sensory information. J. Mot. Behav. 34, 291–298 (2002).
Repp, B.H. Perception of timing is more context sensitive than sensorimotor synchronization. Percept. Psychophys. 64, 703–716 (2002).
Billon, M. & Semjen, A. The timing effects of accent production in synchronization and continuation tasks performed by musicians and nonmusicians. Psychol. Res. 58, 206–217 (1995).
Billon, M., Semjen, A. & Stelmach, G.E. The timing effects of accent production in periodic finger-tapping sequences. J. Mot. Behav. 28, 198–210 (1996).
Clegg, B.A., DiGirolamo, G.J. & Keele, S.W. Sequence learning. Trends Cogn. Sci. 2, 275–281 (1998).
Shin, J.C. & Ivry, R.B. Concurrent learning of temporal and spatial sequences. J. Exp. Psychol. Learn. Mem. Cogn. 28, 445–457 (2002).
Jones, M.R. Dynamic pattern structure in music - recent theory and research. Percept. Psychophys. 41, 621–634 (1987).
Jones, M.R., Boltz, M. & Kidd, G. Controlled attending as a function of melodic and temporal context. Percept. Psychophys. 32, 211–218 (1982).
Boltz, M.G. The generation of temporal and melodic expectancies during musical listening. Percept. Psychophys. 53, 585–600 (1993).
Schmuckler, M.A. & Boltz, M.G. Harmonic and rhythmic influences on musical expectancy. Percept. Psychophys. 56, 313–325 (1994).
Schellenberg, E.G., Krysciak, A.M. & Campbell, R.J. Perceiving emotion in melody: interactive effects of pitch and rhythm. Music Percept. 18, 155–171 (2000).
Yee, W., Holleran, S. & Jones, M.R. Sensitivity to event timing in regular and irregular sequences: influences of musical skill. Percept. Psychophys. 56, 461–471 (1994).
Barnes, R. & Jones, M.R. Expectancy, attention and time. Cognit. Psychol. 41, 254–311 (2000).
Jones, M.R., Moynihan, H., MacKenzie, N. & Puente, J. Temporal aspects of stimulus-driven attending in dynamic arrays. Psychol. Sci. 13, 313–319 (2002).
Jones, M.R. & Boltz, M. Dynamic attending and responses to time. Psychol. Rev. 96, 459–491 (1989).
Large, E.W. & Jones, M.R. The dynamics of attending: how people track time-varying events. Psychol. Rev. 106, 119–159 (1999).
Jones, M.R. Time, our lost dimension - toward a new theory of perception, attention and memory. Psychol. Rev. 83, 323–355 (1976).
Drake, C., Jones, M.R. & Baruch, C. The development of rhythmic attending in auditory sequences: attunement, referent period, focal attending. Cognition 77, 251–288 (2000).
Jones, M.R. & Pfordresher, P.Q. Tracking musical patterns using joint accent structure. Can. J. Exp. Psychol. 51, 271–291 (1997).
Bapi, R.S., Doya, K. & Harner, A.M. Evidence for effector independent and dependent representations and their differential time course of acquisition during motor sequence learning. Exp. Brain Res. 132, 149–162 (2000).
Hazeltine, E. The representational nature of sequence learning: evidence for goal-based codes. in Common Mechanisms in Perception and Action. Attention and Performance XIX (eds. Prinz, W. & Hommel, B.) (Oxford Univ. Press, Oxford, 2002).
Smith, G.J. Teaching a long sequence of behavior using whole task training, forward chaining, and backward chaining. Percept. Mot. Skills 89, 951–965 (1999).
Ghahramani, Z. & Wolpert, D.M. Modular decomposition in visuomotor learning. Nature 386, 392–395 (1997).
Sloboda, J.A. Visual perception of musical notation: registering pitch symbols in memory. Q. J. Exp. Psychol. 28, 1–16 (1976).
Keele, S.W. et al. On the modularity of sequence representation. J. Mot. Behav. 27, 17–30 (1995).
Koch, I. & Hoffmann, J. Patterns, chunks, and hierarchies in serial reaction-time tasks. Psychol. Res. 63, 22–35 (2000).
Povel, D.J. & Collard, R. Structural factors in patterned finger tapping. Acta Psychologica 52, 107–123 (1982).
Stadler, M.A. Implicit serial learning: questions inspired by Hebb (1961). Mem. Cognit. 21, 819–827 (1993).
Drake, C. Psychological processes involved in the temporal organization of complex auditory sequences: Universal and acquired processes. Music Percept. 16, 11–26 (1998).
Münte, T.F., Altenmüller, E. & Jäncke, L. The musician's brain as a model of neuroplasticity. Nat. Rev. Neurosci. 3, 473–478 (2002).
Macar, F. et al. Activation of the supplementary motor area and of attentional networks during temporal processing. Exp. Brain Res. 142, 475–485 (2002).
Schubotz, R.I., Friederici, A.D. & von Cramon, D.Y. Time perception and motor timing: a common cortical and subcortical basis revealed by fMRI. Neuroimage 11, 1–12 (2000).
Penhune, V.B., Zatorre, R.J. & Evans, A.C. Cerebellar contributions to motor timing: a PET study of auditory and visual rhythm reproduction. J. Cogn. Neurosci. 10, 752–765 (1998).
Sakai, K., Ramnani, N. & Passingham, R.E. Learning of sequences of finger movements and timing: frontal lobe and action-oriented representation. J. Neurophysiol. 88, 2035–2046 (2002).
Sakai, K. et al. What and when: parallel and convergent processing in motor control. J. Neurosci. 20, 2691–2700 (2000).
Schubotz, R.I. & von Cramon, D.Y. Interval and ordinal properties of sequences are associated with distinct premotor areas. Cereb. Cortex 11, 210–222 (2001).
Schubotz, R.I. & von Cramon, D.Y. Predicting perceptual events activates corresponding motor schemes in lateral premotor cortex: an fMRI study. Neuroimage 15, 787–796 (2002).
Sergent, J., Zuck, E., Terriah, S. & Macdonald, B. Distributed neural network underlying musical sight-reading and keyboard performance. Science 257, 106–109 (1992).
Schön, D., Anton, J.L., Roth, M. & Besson, M. An fMRI study of music sight-reading. Neuroreport 13, 2285–2289 (2002).
Satoh, M., Takeda, K., Nagata, K., Hatazawa, J. & Kuzuhara, S. Activated brain regions in musicians during an ensemble: a PET study. Brain Res. Cogn. Brain Res. 12, 101–108 (2001).
Janata, P., Tillmann, B. & Bharucha, J.J. Listening to polyphonic music recruits domain-general attention and working memory circuits. Cogn. Aff. Behav. Neurosci. 2, 121–140 (2002).
Tillmann, B., Janata, P. & Bharucha, J.J. Activation of the inferior frontal cortex in musical priming. Cogn. Brain Res. 16, 145–161 (2003).
Janata, P. et al. The cortical topography of tonal structures underlying Western music. Science 298, 2167–2170 (2002).
Zatorre, R.J., Evans, A.C. & Meyer, E. Neural mechanisms underlying melodic perception and memory for pitch. J. Neurosci. 14, 1908–1919 (1994).
Zatorre, R.J., Halpern, A.R., Perry, D.W., Meyer, E. & Evans, A.C. Hearing in the mind's ear: a PET investigation of musical imagery and perception. J. Cogn. Neurosci. 8, 29–46 (1996).
Koelsch, S. et al. Bach speaks: a cortical “language-network” serves the processing of music. Neuroimage 17, 956–966 (2002).
Halpern, A.R. & Zatorre, R.J. When that tune runs through your head: a PET investigation of auditory imagery for familiar melodies. Cereb. Cortex 9, 697–704 (1999).
Langheim, F.J.P., Callicott, J.H., Mattay, V.S., Duyn, J.H. & Weinberger, D.R. Cortical systems associated with covert music rehearsal. Neuroimage 16, 901–908 (2002).
Nobre, A.C. The attentive homunculus: now you see it, now you don't. Neurosci. Biobehav. Rev. 25, 477–496 (2001).
Coull, J.T., Frith, C.D., Buchel, C. & Nobre, A.C. Orienting attention in time: behavioral and neuroanatomical distinction between exogenous and endogenous shifts. Neuropsychologia 38, 808–819 (2000).
Coull, J.T. & Nobre, A.C. Where and when to pay attention: the neural systems for directing attention to spatial locations and to time intervals as revealed by both PET and fMRI. J. Neurosci. 18, 7426–7435 (1998).
Meck, W.H. & Benson, A.M. Dissecting the brain's internal clock: how frontal-striatal circuitry keeps time and shifts attention. Brain Cogn. 48, 195–211 (2002).
Kermadi, I., Jurquet, Y., Arzi, M. & Joseph, J.P. Neural activity in the caudate nucleus of monkeys during spatial sequencing. Exp. Brain Res. 94, 352–356 (1993).
Kermadi, I. & Joseph, J.P. Activity in the caudate nucleus of monkey during spatial sequencing. J. Neurophysiol. 74, 911–933 (1995).
Graybiel, A.M. Building action repertoires: memory and learning functions of the basal ganglia. Curr. Opin. Neurobiol. 5, 733–741 (1995).
Graybiel, A.M. The basal ganglia and chunking of action repertoires. Neurobiol. Learn. Mem. 70, 119–136 (1998).
Jog, M.S., Kubota, Y., Connolly, C.I., Hillegaart, V. & Graybiel, A.M. Building neural representations of habits. Science 286, 1745–1749 (1999).
Jones, M.R. Attending to auditory events: the role of temporal organization. in Thinking in Sound: The Cognitive Psychology of Human Audition (eds. McAdams, S. & Bigand, E.) 69–112 (Oxford Univ. Press, Oxford, 1993).
Acknowledgements
We thank R. Ivry for comments on an earlier version of this manuscript. Supported by US National Institutes of Health grants P50 NS17778-18, R03 DC05146 and NS33504-10.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Janata, P., Grafton, S. Swinging in the brain: shared neural substrates for behaviors related to sequencing and music. Nat Neurosci 6, 682–687 (2003). https://doi.org/10.1038/nn1081
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1081
This article is cited by
-
The role of auditory feedback in the motor learning of music in experienced and novice performers
Scientific Reports (2022)
-
The Effects of an Integrated Programme on Developing Fundamental Movement Skills and Rhythmic Abilities in Early Childhood
Early Childhood Education Journal (2020)
-
Spontaneous synchronization to speech reveals neural mechanisms facilitating language learning
Nature Neuroscience (2019)
-
Alteration of perceived emotion and brain functional connectivity by changing the musical rhythmic pattern
Experimental Brain Research (2019)
-
Systemic functional adaptedness and domain-general cognition: broadening the scope of evolutionary psychology
Biology & Philosophy (2019)