Abstract
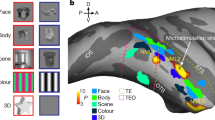
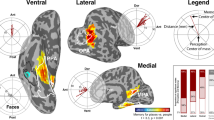
The organizing principles that govern the layout of human object-related areas are largely unknown. Here we propose a new organizing principle in which object representations are arranged according to a central versus peripheral visual field bias. The proposal is based on the finding that building-related regions overlap periphery-biased visual field representations, whereas face-related regions are associated with center-biased representations. Furthermore, the eccentricity maps encompass essentially the entire extent of object-related occipito-temporal cortex, indicating that most object representations are organized with respect to retinal eccentricity. A control experiment ruled out the possibility that the results are due exclusively to unequal feature distribution in these images. We hypothesize that brain regions representing object categories that rely on detailed central scrutiny (such as faces) are more strongly associated with processing of central information, compared to representations of objects that may be recognized by more peripheral information (such as buildings or scenes).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Yarbus, A. L. Eye Movements and Vision (Plenum, New York, 1967).
Gattass, R., Gross, C. G. & Sandell, J. H. Visual topography of V2 in the macaque. J. Comp. Neurol. 201, 519–539 (1981).
Gattass, R., Sousa, A. P. & Gross, C. G. Visuotopic organization and extent of V3 and V4 of the macaque. J. Neurosci. 8, 1831–1845 (1988).
DeYoe, E. A. et al. Mapping striate and extrastriate visual areas in human cerebral cortex. Proc. Natl. Acad. Sci. USA 93, 2382–2386 (1996).
Tootell, R. B. H. et al. Functional analysis of V3A and related areas in human visual cortex. J. Neurosci. 71, 7060–7078 (1997).
Engel, S. A., Glover, G. H. & Wandell, B. A. Retinotopic organization in human visual cortex and the spatial precision of functional MRI. Cereb. Cortex 7, 181–192 (1997).
Sereno, M. I. et al. Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science 268, 889–893 (1995).
Rosa, M. G. in Extrastriate Cortex in Primates (eds. Rockland, K. S., Kaas, J. & Peters, A.) 127–203 (Plenum, New York, 1997).
Desimone, R. & Ungerleider, L. G. Multiple visual areas in the caudal superior temporal sulcus of the macaque. J. Comp. Neurol. 248, 164–189 (1986).
Boussaoud, D., Desimone, R. & Ungerleider, L. G. Visual topography of area TEO in the macaque. J. Comp. Neurol. 306, 554–575 (1991).
Aguirre, G. K., Zarahn, E. & D'Esposito, M. An area within human ventral cortex sensitive to “building” stimuli: evidence and implications. Neuron 21, 373–383 (1998).
Epstein, R. & Kanwisher, N. A cortical representation of the local visual environment. Nature 392, 598–601 (1998).
Ishai, A., Ungerleider, L. G., Martin, A., Schouten, H. L. & Haxby, J. V. Distributed representation of objects in the human ventral visual pathway. Proc. Natl. Acad. Sci. USA 96, 9379–9384 (1999).
Puce, A., Allison, T., Asgaei, M., Gore, J. C. & McCarthy, G. Differential sensitivity of human visual cortex to faces, letterstrings and textures: a functional magnetic resonance imaging study. J. Neurosci. 16, 5205–5215 (1996).
Clark, V. P. et al. Functional magnetic resonance imaging of human visual cortex during face matching: a comparison with positron emission tomography. Neuroimage 4, 1–15 (1996).
Kanwisher, N., McDermott, J. & Chun, M. M. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J. Neurosci. 17, 4302–4311 (1997).
McCarthy, G., Puce, A., Gore, J. C. & Allison, T. Face specific processing in the human fusiform gyrus. J. Cogn. Neurosci. 9, 605–610 (1997).
Halgren, E. et al. Location of human face-selective cortex with respect to retinotopic areas. Hum. Brain Mapp. 7, 29–37 (1999).
Epstein, R., Harris, A., Stanley, D. & Kanwisher, N. The parahippocampal place area: recognition, navigation, or encoding? Neuron 23, 115–125 (1999).
Grill-Spector, K. et al. A sequence of object-processing stages revealed by fMRI in the human occipital lobe. Hum. Brain Mapp. 6, 316–328 (1998).
Engel, S. A. et al. fMRI of human visual cortex. Nature 369, 525 (1994).
Hadjikhani, N., Liu, A. K., Dale, A. M., Cavanagh, P. & Tootell, R. B. Retinotopy and color sensitivity in human visual cortical area V8. Nat. Neurosci. 1, 235–241 (1998).
Talairach, J. & Tournoux, P. Co-Planar Stereotaxic Atlas of the Human Brain (Thieme Medical, New York, 1988).
Haxby, J. V. et al. The effect of face inversion on activity in human neural systems for face and object perception. Neuron 22, 189–199 (1999).
Gauthier, I., Tarr, M. J., Anderson, A. W., Skudlarski, P. & Gore, J. C. Activation of the middle fusiform 'face area' increases with expertise in recognizing novel objects. Nat. Neurosci. 2, 568–573 (1999).
Malach, R. et al. Object-related activity revealed by functional magnetic resonance imaging in human occipital cortex. Proc. Natl. Acad. Sci. USA 92, 8135–8139 (1995).
Hoffman, E. A. & Haxby, J. V. Distinct representations of eye gaze and identity in the distributed human neural system for face perception. Nat. Neurosci. 3, 80–84 (2000).
Grill-Spector, K. et al. Differential processing of objects under various viewing conditions in the human lateral occipital complex. Neuron 24, 187–203 (1999).
Tanaka, K. Neuronal mechanisms of object recognition. Science 262, 685–688 (1993).
Tanaka, K. Inferotemporal cortex and object vision. Annu. Rev. Neurosci. 19, 109–139 (1996).
Gross, C. G., Bender, D. B. & Rocha-Miranda, C. E. Visual receptive fields of neurons in inferotemporal cortex of the monkey. Science 166, 1303–1306 (1969).
Seltzer, B. & Pandya, D. N. Afferent cortical connections and architectonics of the superior temporal sulcus and surrounding cortex in the rhesus monkey. Brain Res. 149, 1–24 (1978).
Desimone, R., Fleming, J. & Gross, C. G. Prestriate afferents to inferior temporal cortex: an HRP study. Brain Res. 184, 41–55 (1980).
Baizer, J. S., Ungerleider, L. G. & Desimone, R. Organization of visual inputs to the inferior temporal and posterior parietal cortex in macaques. J. Neurosci. 11, 168–190 (1991).
Hikosaka, K. Representation of foveal visual fields in the ventral bank of the superior temporal sulcus in the posterior inferotemporal cortex of the macaque monkey. Behav. Brain Res. 96, 101–113 (1998).
Gauthier, I., Skudlarski, P., Gore, J. C. & Anderson, A. W. Expertise for cars and birds recruits brain areas involved in face recognition. Nat. Neurosci. 3, 191–197 (2000).
Gauthier, I. et al. The fusiform “face area” is part of a network that processes faces at the individual level. J. Cogn. Neurosci. 12, 495–504 (2000).
Tootell, R.B. & Hadjikani, N. Where is 'dorsal v4' in human visual cortex? retinopic, topagraphic and functional evidence. Cereb. Cortex 11, 298–311 (2001).
Grill-Spector, K., Kushnir, T., Hendler, T. & Malach, R. The dynamics of object-selective activation correlate with recognition performance in humans. Nat. Neurosci. 3, 837–843 (2000).
Grill-Spector, K., Kushnir, T., Edelman, S., Itzchak, Y. & Malach, R. Cue-invariant activation in object-related areas of the human occipital lobe. Neuron 21, 191–202 (1998).
Friston, J. et al. Statistical parametric maps in functional imaging: a general linear approach. Hum. Brain Mapp. 2, 189–210 (1995).
Acknowledgements
This study was funded by JSMF 99-28 CN-QUA.05 and Israel Academy 8009 grants. We thank M. Harel for help with the brain flattening, E. Okon for technical help, and V. Levi, S. Peled, D. Ben Bashat, P. Rotshtein and D. Palti for help with running the experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Levy, I., Hasson, U., Avidan, G. et al. Center–periphery organization of human object areas. Nat Neurosci 4, 533–539 (2001). https://doi.org/10.1038/87490
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/87490
This article is cited by
-
EEG Differences in the Perception of Own and Others’ Faces: Application of a Comprehensive Approach to the Analysis of EEG Data
Neuroscience and Behavioral Physiology (2023)
-
The spatiotemporal neural dynamics of object location representations in the human brain
Nature Human Behaviour (2022)
-
Layer-specific, retinotopically-diffuse modulation in human visual cortex in response to viewing emotionally expressive faces
Nature Communications (2022)
-
Direct comparison of contralateral bias and face/scene selectivity in human occipitotemporal cortex
Brain Structure and Function (2022)
-
Differential spatial computations in ventral and lateral face-selective regions are scaffolded by structural connections
Nature Communications (2021)