Abstract
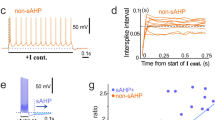
Potassium leak channels are essential to neurophysiological function. Leaks suppress excitability through maintenance of resting membrane potential below the threshold for action potential firing. Conversely, voltage-dependent potassium channels permit excitation because they do not interfere with rise to threshold, and they actively promote recovery and rapid re-firing. Previously attributed to distinct transport pathways, we demonstrate here that phosphorylation of single, native hippocampal and cloned KCNK2 potassium channels produces reversible interconversion between leak and voltage-dependent phenotypes. The findings reveal a pathway for dynamic regulation of excitability.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Goldman, D. E. Potential, impedance, and rectification in membranes. J. Gen. Physiol. 27, 37–60 (1943).
Hodgkin, A. L. & Katz, B. The effect of sodium ions on the electrical activity of the giant axon of the squid. J. Physiol. (Lond.) 108, 37–77 (1949).
Hodgkin, A. L. & Huxely, A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. (Lond.) 117, 500–544 (1952).
Hille, B. Ionic selectivity of Na and K channels of nerve membranes. Membranes 3, 255–323 (1975).
Adams, D. J., Smith, S. J. & Thompson, S. H. Ionic currents in molluscan soma. Annu. Rev. Neurosci. 3, 141–167 (1980).
Schmidt, H. & Stampfli, R. The effect of tetraethylammonium chloride on single Ranvier's nodes. Pflugers Arch. Gesamte Physiol. Menschen Tiere 287, 311–325 (1966).
Hille, B. Potassium channels in myelinated nerve. Selective permeability to small cations. J. Gen. Physiol. 61, 669–686 (1973).
Baker, M., Bostock, H., Grafe, P. & Martius, P. Function and distribution of three types of rectifying channel in rat spinal root myelinated axons. J. Physiol. (Lond.) 383, 45–67 (1987).
Chang, D. C. Is the K+ permeability of the resting membrane controlled by the excitable K+ channel? Biophys. J. 50, 1095–1100 (1986).
Jones, S. W. On the resting potential of isolated frog sympathetic neurons. Neuron 3, 153–161 (1989).
Backx, P. H. & Marban, E. Background potassium current active during the plateau of the action potential in guinea pig ventricular myocytes. Circ. Res. 72, 890–900 (1993).
Siegelbaum, S. A., Camardo, J. S. & Kandel, E. R. Serotonin & cyclic AMP close single K+ channels in Aplysia sensory neurones. Nature 299, 413–417 (1982).
Shen, K. Z., North, R. A. & Surprenant, A. Potassium channels opened by noradrenaline and other transmitters in excised membrane patches of guinea-pig submucosal neurones. J. Physiol. (Lond.) 445, 581–599 (1992).
Buckler, K. J. A novel oxygen-sensitive potassium current in rat carotid body type I cells. J. Physiol.(Lond.) 498, 649–662 (1997).
Ketchum, K. A., Joiner, W. J., Sellers, A. J., Kaczmarek, L. K. & Goldstein, S. A. N. A new family of outwardly-rectifying potassium channel proteins with two pore domains in tandem. Nature 376, 690–695 (1995).
Goldstein, S. A. N., Price, L. A., Rosenthal, D. N. & Pausch, M. H. ORK1, a potassium-selective leak channel with two pore domains cloned from Drosophila melanogaster by expression in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 93, 13256–13261 (1996).
Goldstein, S. A. N., Bockenhauer, D., O'Kelly, I. & Zilberberg, N. Potassium leak channels with 2 P domains. Nat. Reviews Neurosci. 2, 175–184 (2001).
Goldstein, S. A. N., Wang, K. W., Ilan, N. & Pausch, M. Sequence and function of the two P domain potassium channels: implications of an emerging superfamily. J. Molec. Med. 76, 13–20 (1998).
Patel, A. J. et al. A mammalian two pore domain mechano-gated S-like K+ channel. EMBO J. 17, 4283–4290 (1998).
Maingret, F. et al. TREK-1 is a heat-activated background K+ channel. EMBO J. 19, 2483–2491 (2000).
Maingret, F., Patel, A. J., Lesage, F., Lazdunski, M. & Honore, E. Lysophospholipids open the two-pore domain mechano-gated K+ channels TREK-1 and TRAAK. J. Biol. Chem. 275, 10128–10133 (2000).
Fink, M. et al. Cloning, functional expression and brain localization of a novel unconventional outward rectifier K+ channel. EMBO J. 15, 6854–6862 (1996).
Meadows, H. J. et al. Cloning, localisation and functional expression of the human orthologue of the TREK-1 potassium channel. Eur. J. Physiol. 439, 714–722 (2000).
Eves, E. M. et al. Immortal rat hippocampal cell lines exhibit neuronal and glial lineages and neurotrophin gene expression. Proc. Natl. Acad. Sci. USA 89, 4373–4377 (1992).
Zilberberg, N., Ilan, N., Gonzalez-Colaso, R. & Goldstein, S. A. N. Opening and closing of KCNK0 potassium leak channels is tightly-regulated. J. Gen. Physiol. 116, 721–734 (2000).
Zhou, Y. et al. A dynamically regulated 14-3-3. Slob, and slowpoke potassium channel complex in Drosophila presynaptic nerve terminals. Neuron 22, 809–818 (1999).
Vandenberg, C. A. Inward rectification of a potassium channel in cardiac ventricular cells depends on internal magnesium ions. Proc. Natl. Acad. Sci. USA 84, 2560–2564 (1987).
Kubo, Y., Reuveny, E., Slesinger, P. A., Jan, Y. N. & Jan, L. Y. Primary structure and functional expression of a rat G-protein-coupled muscarinic potassium channel. Nature 364, 802–806 (1993).
Lopatin, A. N., Makhina, E. N. & Nichols, C. G. Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature 372, 366–369 (1994).
Fakler, B. et al. Strong voltage-dependent inward-rectification of inward-rectifier K+ channels is caused by intracellular spermine. Cell 80, 149–154 (1995).
Ahmed, A. et al. A molecular target for viral killer toxin: TOK1 potassium channels. Cell 99, 283–291 (1999).
Vergani, P., Miosga, T., Jarvis, S. M. & Blatt, M. R. Extracellular K+ and Ba2+ mediate voltage-dependent inactivation of the outward-rectifying K+ channel encoded by the yeast gene TOK1. FEBS Lett. 405, 337–344 (1997).
Vergani, P., Hamilton, D., Jarvis, S. & Blatt, M. R. Mutations in the pore regions of the yeast K+ channel YKC1 affect gating by extracellular K+. EMBO J. 17, 7190–7198 (1998).
Loukin, S. H. et al. Random mutagenesis reveals a region important for gating of the yeast K+ channel Ykc1. EMBO J. 16, 4817–4825 (1997).
Loukin, S. H. & Saimi, Y. K+-dependent composite gating of the yeast K+ channel, Tok1. Biophys. J. 77, 3060–3070 (1999).
Sigworth, F. J. Voltage gating of ion channels. Q. Rev. Biophys. 27, 1–40 (1994).
Yellen, G. The moving parts of voltage-gated ion channels. Q. Rev. Biophys. 31, 239–295 (1998).
Bean, B. P. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature 340, 153–156 (1989).
Colecraft, H. M., Patil, P. G. & Yue, D. T. Differential occurrence of reluctant openings in G-protein-inhibited N- and P/Q-type calcium channels. J. Gen. Physiol. 115, 175–192 (2000).
Ilan, N. & Goldstein, S. A. N. KCNK0: single, cloned potassium leak channels are multi-ion pores. Biophys. J. 80, 241–254 (2001).
McCormick, D. A. & Bal, T. Sleep and arousal: thalamocortical mechanisms. Annu. Rev. Neurosci. 20, 185–215 (1997).
Millar, J. A. et al. A functional role for the two-pore domain potassium channel TASK-1 in cerebellar granule neurons. Proc. Natl. Acad. Sci. USA 97, 3614–3618 (2000).
Talley, E. M., Lei, Q. B., Sirois, J. E. & Bayliss, D. A. TASK-1, a two-pore domain K+ channel, is modulated by multiple neurotransmitters in motoneurons. Neuron 25, 399–410 (2000).
Sirois, J. E., Lei, Q., Talley, E. M., Lynch, C. III & Bayliss, D. A. The TASK-1 two-pore domain K+ channel is a molecular substrate for neuronal effects of inhalation anesthetics. J. Neurosci. 20, 6347–6354 (2000).
Kim, Y., Bang, H. & Kim, D. TASK-3, a new member of the tandem pore K+ channel family. J. Biol. Chem. 275, 9340–9347 (2000).
Colquhoun, D. & Sigworth, F. J. in Single-Channel Recording (eds. B. Sakmann & E. Neher) 483–588 (Plenum, New York, 1995).
Acknowledgements
This work was supported by grants to S.A.N.G. from the National Institutes of Health. D.B. is supported by the Child Health Research Center (Yale University) and an award from the National Institute of Diabetes and Digestive and Kidney Diseases. We thank F. Sesti and R. Goldstein for discussions and advice during the course of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bockenhauer, D., Zilberberg, N. & Goldstein, S. KCNK2: reversible conversion of a hippocampal potassium leak into a voltage-dependent channel. Nat Neurosci 4, 486–491 (2001). https://doi.org/10.1038/87434
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/87434
This article is cited by
-
Altered detrusor contractility and voiding patterns in mice lacking the mechanosensitive TREK-1 channel
BMC Urology (2019)
-
Voltage gating of mechanosensitive PIEZO channels
Nature Communications (2018)
-
Grafting voltage and pharmacological sensitivity in potassium channels
Cell Research (2016)
-
Much more than a leak: structure and function of K2P-channels
Pflügers Archiv - European Journal of Physiology (2015)
-
Two-pore domain potassium channels: potential therapeutic targets for the treatment of pain
Pflügers Archiv - European Journal of Physiology (2015)