Abstract
The identification of distinct cell types in the basal ganglia has been critical to our understanding of basal ganglia function and the treatment of neurological disorders. The external globus pallidus (GPe) is a key contributor to motor suppressing pathways in the basal ganglia, yet its neuronal heterogeneity has remained an untapped resource for therapeutic interventions. Here we demonstrate that optogenetic interventions that dissociate the activity of two neuronal populations in the GPe, elevating the activity of parvalbumin (PV)-expressing GPe neurons over that of Lim homeobox 6 (Lhx6)-expressing GPe neurons, restores movement in dopamine-depleted mice and attenuates pathological activity of basal ganglia output neurons for hours beyond stimulation. These results establish the utility of cell-specific interventions in the GPe to target functionally distinct pathways, with the potential to induce long-lasting recovery of movement despite the continued absence of dopamine.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Saunders, A. et al. A direct GABAergic output from the basal ganglia to frontal cortex. Nature 521, 85–89 (2015).
Mastro, K.J., Bouchard, R.S., Holt, H.A. & Gittis, A.H. Transgenic mouse lines subdivide external segment of the globus pallidus (GPe) neurons and reveal distinct GPe output pathways. J. Neurosci. 34, 2087–2099 (2014).
Kita, H. Globus pallidus external segment. Prog. Brain Res. 160, 111–133 (2007).
Bevan, M.D., Magill, P.J., Terman, D., Bolam, J.P. & Wilson, C.J. Move to the rhythm: oscillations in the subthalamic nucleus-external globus pallidus network. Trends Neurosci. 25, 525–531 (2002).
Mallet, N. et al. Parkinsonian beta oscillations in the external globus pallidus and their relationship with subthalamic nucleus activity. J. Neurosci. 28, 14245–14258 (2008).
Bergman, H. et al. Physiological aspects of information processing in the basal ganglia of normal and parkinsonian primates. Trends Neurosci. 21, 32–38 (1998).
Hernández, V.M. et al. Parvalbumin+ neurons and Npas1+ neurons are distinct neuron classes in the mouse external globus pallidus. J. Neurosci. 35, 11830–11847 (2015).
Dodson, P.D. et al. Distinct developmental origins manifest in the specialized encoding of movement by adult neurons of the external globus pallidus. Neuron 86, 501–513 (2015).
Abdi, A. et al. Prototypic and arkypallidal neurons in the dopamine-intact external globus pallidus. J. Neurosci. 35, 6667–6688 (2015).
Mallet, N. et al. Dichotomous organization of the external globus pallidus. Neuron 74, 1075–1086 (2012).
Hegeman, D.J., Hong, E.S., Hernández, V.M. & Chan, C.S. The external globus pallidus: progress and perspectives. Eur. J. Neurosci. 43, 1239–1265 (2016).
Oh, Y.M. et al. Using a novel PV-Cre rat model to characterize pallidonigral cells and their terminations. Brain Struct. Funct. http://dx.doi.org/10.1007/s00429-016-1346-2 (2016).
Albin, R.L., Young, A.B. & Penney, J.B. The functional anatomy of basal ganglia disorders. Trends Neurosci. 12, 366–375 (1989).
DeLong, M.R. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 13, 281–285 (1990).
Kravitz, A.V. et al. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature 466, 622–626 (2010).
Gittis, A.H. et al. New roles for the external globus pallidus in basal ganglia circuits and behavior. J. Neurosci. 34, 15178–15183 (2014).
Herrera, A.J., Castaño, A., Venero, J.L., Cano, J. & Machado, A. The single intranigral injection of LPS as a new model for studying the selective effects of inflammatory reactions on dopaminergic system. Neurobiol. Dis. 7, 429–447 (2000).
Mallet, N. et al. Arkypallidal cells send a stop signal to striatum. Neuron 89, 308–316 (2016).
Wang, J. et al. Coordinated reset deep brain stimulation of subthalamic nucleus produces long-lasting, dose-dependent motor improvements in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine non-human primate model of parkinsonism. Brain Stimul. 9, 609–617 (2016).
Soares, J. et al. Role of external pallidal segment in primate parkinsonism: comparison of the effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism and lesions of the external pallidal segment. J. Neurosci. 24, 6417–6426 (2004).
Rubin, J.E., McIntyre, C.C., Turner, R.S. & Wichmann, T. Basal ganglia activity patterns in parkinsonism and computational modeling of their downstream effects. Eur. J. Neurosci. 36, 2213–2228 (2012).
Gerfen, C.R. et al. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 250, 1429–1432 (1990).
Smith, Y., Bevan, M.D., Shink, E. & Bolam, J.P. Microcircuitry of the direct and indirect pathways of the basal ganglia. Neuroscience 86, 353–387 (1998).
Hammond, C., Bergman, H. & Brown, P. Pathological synchronization in Parkinson's disease: networks, models and treatments. Trends Neurosci. 30, 357–364 (2007).
Kühn, A.A., Kupsch, A., Schneider, G.H. & Brown, P. Reduction in subthalamic 8–35 Hz oscillatory activity correlates with clinical improvement in Parkinson's disease. Eur. J. Neurosci. 23, 1956–1960 (2006).
Weinberger, M. et al. Beta oscillatory activity in the subthalamic nucleus and its relation to dopaminergic response in Parkinson's disease. J. Neurophysiol. 96, 3248–3256 (2006).
Vitek, J.L., Zhang, J., Hashimoto, T., Russo, G.S. & Baker, K.B. External pallidal stimulation improves parkinsonian motor signs and modulates neuronal activity throughout the basal ganglia thalamic network. Exp. Neurol. 233, 581–586 (2012).
Bernheimer, H., Birkmayer, W., Hornykiewicz, O., Jellinger, K. & Seitelberger, F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J. Neurol. Sci. 20, 415–455 (1973).
Riederer, P. & Wuketich, S. Time course of nigrostriatal degeneration in Parkinson's disease. A detailed study of influential factors in human brain amine analysis. J. Neural Transm. 38, 277–301 (1976).
Betarbet, R., Sherer, T.B. & Greenamyre, J.T. Animal models of Parkinson's disease. Bioessays 24, 308–318 (2002).
Deumens, R., Blokland, A. & Prickaerts, J. Modeling Parkinson's disease in rats: an evaluation of 6-OHDA lesions of the nigrostriatal pathway. Exp. Neurol. 175, 303–317 (2002).
Fahn, S. Description of Parkinson's disease as a clinical syndrome. Ann. NY Acad. Sci. 991, 1–14 (2003).
Tass, P.A. et al. Coordinated reset has sustained aftereffects in Parkinsonian monkeys. Ann. Neurol. 72, 816–820 (2012).
Adamchic, I. et al. Coordinated reset neuromodulation for Parkinson's disease: proof-of-concept study. Mov. Disord. 29, 1679–1684 (2014).
Corbit, V.L. et al. Pallidostriatal projections promote β oscillations in a dopamine-depleted biophysical network model. J. Neurosci. 36, 5556–5571 (2016).
Holgado, A.J., Terry, J.R. & Bogacz, R. Conditions for the generation of beta oscillations in the subthalamic nucleus-globus pallidus network. J. Neurosci. 30, 12340–12352 (2010).
Erez, Y., Czitron, H., McCairn, K., Belelovsky, K. & Bar-Gad, I. Short-term depression of synaptic transmission during stimulation in the globus pallidus of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated primates. J. Neurosci. 29, 7797–7802 (2009).
Bar-Gad, I., Elias, S., Vaadia, E. & Bergman, H. Complex locking rather than complete cessation of neuronal activity in the globus pallidus of a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated primate in response to pallidal microstimulation. J. Neurosci. 24, 7410–7419 (2004).
Chin, G.D. & Hutchison, W.D. Effects of cobalt and bicuculline on focal microstimulation of rat pallidal neurons in vivo. Brain Stimul. 1, 134–150 (2008).
Bugaysen, J., Bar-Gad, I. & Korngreen, A. The impact of stimulation induced short-term synaptic plasticity on firing patterns in the globus pallidus of the rat. Front. Syst. Neurosci. 5, 16 (2011).
Johnson, M.D., Zhang, J., Ghosh, D., McIntyre, C.C. & Vitek, J.L. Neural targets for relieving parkinsonian rigidity and bradykinesia with pallidal deep brain stimulation. J. Neurophysiol. 108, 567–577 (2012).
Chan, C.S. et al. HCN channelopathy in external globus pallidus neurons in models of Parkinson's disease. Nat. Neurosci. 14, 85–92 (2011).
Hardman, C.D. & Halliday, G.M. The external globus pallidus in patients with Parkinson's disease and progressive supranuclear palsy. Mov. Disord. 14, 626–633 (1999).
Gong, S. et al. Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J. Neurosci. 27, 9817–9823 (2007).
Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 (2010).
Fogarty, M. et al. Spatial genetic patterning of the embryonic neuroepithelium generates GABAergic interneuron diversity in the adult cortex. J. Neurosci. 27, 10935–10946 (2007).
Gradinaru, V., Mogri, M., Thompson, K.R., Henderson, J.M. & Deisseroth, K. Optical deconstruction of parkinsonian neural circuitry. Science 324, 354–359 (2009).
Kravitz, A.V., Owen, S.F. & Kreitzer, A.C. Optogenetic identification of striatal projection neuron subtypes during in vivo recordings. Brain Res. 1511, 21–32 (2013).
Willard, A.M., Bouchard, R.S. & Gittis, A.H. Differential degradation of motor deficits during gradual dopamine depletion with 6-hydroxydopamine in mice. Neuroscience 301, 254–267 (2015).
Acknowledgements
The authors thank V. Corbit (University of Pittsburgh) and T. Whalen (Carnegie Mellon University) for Matlab analysis scripts and B. Rogowski (Carnegie Mellon University) for surgical support and behavioral video editing. We also thank N. Kessaris (University of College London) and H. Zeng (Allen Institute) for their gifts of the Lhx6-iCre and Pvalb-2A-Cre mice, respectively. This work was supported by NIH grants F31 NS090745-01 (K.M.), F31 NS093944-01 (A.W.) and R00 NS076524, NSF grant DMS 1516288, and grants from the Brain and Behavior Research Foundation (National Alliance for Research on Schizophrenia and Depression Young Investigator Grant), the Parkinson's Disease Foundation, and the NIH Intramural Research Program.
Author information
Authors and Affiliations
Contributions
K.J.M., K.T.Z., and K.H.L. performed behavioral experiments, including the histological verification, and with A.H.G. analyzed the data. K.J.M. and A.M.W. were responsible for the collection and analysis of the in vivo experiments. All authors discussed results and interpretations. K.J.M. and A.H.G. designed the experiments and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
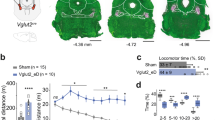
Supplementary Figure 1 Behavioral and pathophysiological symptoms of bilateral DD are apparent within 3–5 d post-depletion.
(a) Schematic of bilateral DD in the medial forebrain bundle (MFB). (b) Quantification of immobility and bradykinesia induced by unilateral (Uni, n = 4) and bilateral (Bi, n = 51) depletions, as compared to dopamine-intact controls (Naive, n = 4). (*p < 0.02, **p < 0.001, Mann Whitney U). (c) Rasters of single units in the GPe of naïve vs. bilateral DD mice. Scale bar, 500 ms. (d) Box plots showing decreases in firing rates (Naive: 44.3 ± 2.6 Hz, n = 73 across 4 animals, versus Acute: 24.6 ± 1.6 Hz,n = 62 across 3 animals, H(1) = 29.775, **p < 0.001, Kruskal-Wallis H test) and (e) increases in coefficients of variation of the interspike intervals (CVISI) (Naïve: 0.63 ± 0.03 versus Acute: 0.80 ± 0.03, H(1) = 22.615, *p < 0.001, Kruskal-Wallis H test) following bilateral DD. Error bars, sem.
Supplementary Figure 2 Histological verification of TH immunoreactivity, viral expression and fiber placements for behavioral optogenetics in global manipulations.
(a) Representative images of striatal TH immunoreactivity in dorsal striatum of healthy dopamine intact tissue compared to a fully depleted bilateral animal. Scale Bar, 200 μm (b) Epifluorescent images of viral expression and fiber identification (yellow arrow) within the GPe. Scale Bar, 500 μm (c) Superimposed traces of viral expression across animals within hSyn-ChR2 (d) Epifluorescent images of viral expression and fiber identification (yellow arrow) with the dorsal striatum. Scale Bar, 500 μm (e) Superimposed traces of viral expression across animals within D1-ChR2 condition. Ctx = Cortex, Str = Striatum, GPe = globus pallidus externa.
Supplementary Figure 3 Histological verification of viral expression and fiber placements for behavioral optogenetics in cell-type manipulations.
(a) Epifluorescent images of viral expression and fiber identification (yellow arrow) within the GPe. Scale Bar, 500 μm (b-f) Superimposed traces of viral expression across animals within PV-ChR2, Lhx6-Arch, CAG-Arch, Lhx6-ChR2 and PV-Arch conditions.
Supplementary Figure 4 PV-ChR2 stimulation induces transient effects in partially and unilaterally depleted mice.
(a) Percentage of time spent in the immobile state before, during and after PV-ChR2 stimulation in lipopolysaccharide (LPS) injected mice. (b) Schematic representation of striatal location for TH analysis with epifluorescent image of a partial depletion of tyrosine hydroxylase. Scale Bar, 200 μm. (c) Quantification of TH levels, normalized to dopamine intact littermate and subsequent categorization into partially and fully depleted animals.(d) Percentage of time spent in the immobile state before, during, and after PV-ChR2 stimulation in partially depleted mice. (e) Overlay of immobility immediately before (pre), during (stim), and after (post) each light pulse. (f) Percent time spent immobile for each animal (grey x = pre, black circle = post) and degree of rescue (red line) as a function of TH remaining. Note sharp cut-off for induction of behavioral rescue at ~20% dopamine remaining. (g) Percentage of time spent in the immobile state before, during, and after PV-ChR2 stimulation in unilaterally depleted mice. (h) Overlay of immobility immediately before (pre), during (stim), and after (post) each light pulse. (i) Percent time spent immobile over the course of the full experimental trial. Error bars, sem.
Supplementary Figure 5 Optical identification using ChR2 and Arch and their corresponding firing properties and waveforms.
(a) Representative responses over 20 trials from a ChR2+ (putative PV) and CHR2- (putative non-PV) neuron responding to 5 ms optical pulses. Yellow bar denotes first significant bin as compared to baseline (b) Firing rate and coefficient of variation of the interspike interval (CVISI) for ChR2+ and ChR2- neurons in dopamine depleted (FR: p = 0.128, CVISI: p = 0.005, Mann Whitney U) (c) Extracellular waveform analysis of the peak-valley ratio and amplitude of individual units identified as ChR2+ (red, closed circles) and ChR2- (black, open circles). Inset: Average waveforms of ChR2+ and ChR2- (Note: nearly complete overlap). Scale bar: 50 μV(vertical), 220 μsec (horizontal) (d) Firing rate and coefficient of variation of the interspike interval (CVISI) for ChR2+ and ChR2- neurons in dopamine intact animals (Naïve) (e) Representative responses over 20 trials from a Arch+ (putative Lhx6) and Arch- (putative non-Lhx6) neuron responding to 1 s optical pulses. (f) Firing rate and CVISI for Arch+ and Arch- neurons (FR: p = 0.990, CV: p = 0.454, Mann Whitney U). (g) Extracellular waveform analysis of the peak-valley ratio and amplitude of individual units identified as Arch+ (blue, closed squares) and Arch- (black, open squares). Inset: Average waveforms of Arch+ and Arch-. Scale bar: 50 μV(vertical), 220 μsec (horizontal).
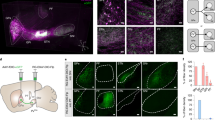
Supplementary Figure 6 PV and Lhx6 overlap partially in the Lhx6-Cre transgenic mouse.
(a) Fluorescent images from the GPe showing overlap between Lhx6-iCre and PV. Left: Lhx6-EYFP neurons. Arrows denote position of PV+ neurons (Purple arrows: Lhx6/PV double labeled; Red arrows: PV-only neurons). Note weaker Lhx6-EYFP expression in Lhx6/PV neurons. Middle: PV+ neurons (Blue arrows: Lhx6-only neurons). Right: Overlay. (b) Box diagram summarizing the proportion of GPe neurons (n = 4194 total neurons across 5 animals) counted that expressed either PV, Lhx6, or both.
Supplementary Figure 7 SNr firing rate is unaltered after PV-ChR2 and Lhx6-Arch, but decreased after hSyn-ChR2 manipulation.
(a) Firing rate of single units collected before (pre) and after (post) stimulation in PV-ChR2 (nPre = 80 vs nPost = 58 units across 3 animals, p = 0.976, Mann Whitney U), hSYn-ChR2 (nPre = 55 vs nPost = 69 unit across 3 animals, p = 0.002, Mann Whitney U) and Lhx6-Arch (nPre = 30 vs nPost = 69 units across 3 animals, p = 0.142, Mann Whitney U).
Supplementary information
Rights and permissions
About this article
Cite this article
Mastro, K., Zitelli, K., Willard, A. et al. Cell-specific pallidal intervention induces long-lasting motor recovery in dopamine-depleted mice. Nat Neurosci 20, 815–823 (2017). https://doi.org/10.1038/nn.4559
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4559
This article is cited by
-
Updating the striatal–pallidal wiring diagram
Nature Neuroscience (2024)
-
Neural signatures of indirect pathway activity during subthalamic stimulation in Parkinson’s disease
Nature Communications (2024)
-
Neuronal and synaptic adaptations underlying the benefits of deep brain stimulation for Parkinson's disease
Translational Neurodegeneration (2023)
-
Cell and circuit complexity of the external globus pallidus
Nature Neuroscience (2023)
-
Dynamical mechanism of parkinsonian beta oscillation in a heterogenous subthalamopallidal network
Nonlinear Dynamics (2023)