Abstract
Tobacco smokers titrate their nicotine intake to avoid its noxious effects, sensitivity to which may influence vulnerability to tobacco dependence, yet mechanisms of nicotine avoidance are poorly understood. Here we show that nicotine activates glucagon-like peptide-1 (GLP-1) neurons in the nucleus tractus solitarius (NTS). The antidiabetic drugs sitagliptin and exenatide, which inhibit GLP-1 breakdown and stimulate GLP-1 receptors, respectively, decreased nicotine intake in mice. Chemogenetic activation of GLP-1 neurons in NTS similarly decreased nicotine intake. Conversely, Glp1r knockout mice consumed greater quantities of nicotine than wild-type mice. Using optogenetic stimulation, we show that GLP-1 excites medial habenular (MHb) projections to the interpeduncular nucleus (IPN). Activation of GLP-1 receptors in the MHb–IPN circuit abolished nicotine reward and decreased nicotine intake, whereas their knockdown or pharmacological blockade increased intake. GLP-1 neurons may therefore serve as 'satiety sensors' for nicotine that stimulate habenular systems to promote nicotine avoidance before its aversive effects are encountered.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Change history
10 April 2017
In the version of this article initially published online, the original source of the Glp1r knockout mice, D.J. Drucker (Lunenfeld Tanenbaum Research Institute, Mount Sinai Hospital, Toronto), was not acknowledged. The error has been corrected in the print, PDF and HTML versions of this article.
References
Kenny, P.J. & Markou, A. Nicotine self-administration acutely activates brain reward systems and induces a long-lasting increase in reward sensitivity. Neuropsychopharmacology 31, 1203–1211 (2006).
Picciotto, M.R. et al. Acetylcholine receptors containing the β2 subunit are involved in the reinforcing properties of nicotine. Nature 391, 173–177 (1998).
Russell, M.A., Wilson, C., Patel, U.A., Feyerabend, C. & Cole, P.V. Plasma nicotine levels after smoking cigarettes with high, medium, and low nicotine yields. BMJ 2, 414–416 (1975).
Fowler, C.D. & Kenny, P.J. Nicotine aversion: neurobiological mechanisms and relevance to tobacco dependence vulnerability. Neuropharmacology 76, 533–544 (2014).
Grill, H.J. & Hayes, M.R. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab. 16, 296–309 (2012).
Jensen, K.P. et al. A CHRNA5 smoking risk variant decreases the aversive effects of nicotine in humans. Neuropsychopharmacology 40, 2813–2821 (2015).
Göke, R., Larsen, P.J., Mikkelsen, J.D. & Sheikh, S.P. Distribution of GLP-1 binding sites in the rat brain: evidence that exendin-4 is a ligand of brain GLP-1 binding sites. Eur. J. Neurosci. 7, 2294–2300 (1995).
Dalle, S., Burcelin, R. & Gourdy, P. Specific actions of GLP-1 receptor agonists and DPP4 inhibitors for the treatment of pancreatic β-cell impairments in type 2 diabetes. Cell. Signal. 25, 570–579 (2013).
Egecioglu, E., Engel, J.A. & Jerlhag, E. The glucagon-like peptide 1 analogue, exendin-4, attenuates the rewarding properties of psychostimulant drugs in mice. PLoS One 8, e69010 (2013).
Graham, D.L., Erreger, K., Galli, A. & Stanwood, G.D. GLP-1 analog attenuates cocaine reward. Mol. Psychiatry 18, 961–962 (2013).
Shirazi, R.H., Dickson, S.L. & Skibicka, K.P. Gut peptide GLP-1 and its analogue, Exendin-4, decrease alcohol intake and reward. PLoS One 8, e61965 (2013).
Campos, R.V., Lee, Y.C. & Drucker, D.J. Divergent tissue-specific and developmental expression of receptors for glucagon and glucagon-like peptide-1 in the mouse. Endocrinology 134, 2156–2164 (1994).
Scott, M.M., Williams, K.W., Rossi, J., Lee, C.E. & Elmquist, J.K. Leptin receptor expression in hindbrain Glp-1 neurons regulates food intake and energy balance in mice. J. Clin. Invest. 121, 2413–2421 (2011).
Mastitskaya, S. et al. Cardioprotection evoked by remote ischaemic preconditioning is critically dependent on the activity of vagal pre-ganglionic neurones. Cardiovasc. Res. 95, 487–494 (2012).
Kang, B.J. et al. Central nervous system distribution of the transcription factor Phox2b in the adult rat. J. Comp. Neurol. 503, 627–641 (2007).
Fowler, C.D. & Kenny, P.J. Intravenous nicotine self-administration and cue-induced reinstatement in mice: effects of nicotine dose, rate of drug infusion and prior instrumental training. Neuropharmacology 61, 687–698 (2011).
Ren, J. et al. Habenula “cholinergic” neurons co-release glutamate and acetylcholine and activate postsynaptic neurons via distinct transmission modes. Neuron 69, 445–452 (2011).
Görlich, A. et al. Reexposure to nicotine during withdrawal increases the pacemaking activity of cholinergic habenular neurons. Proc. Natl. Acad. Sci. USA 110, 17077–17082 (2013).
Ban, K. et al. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation 117, 2340–2350 (2008).
McGehee, D.S., Heath, M.J., Gelber, S., Devay, P. & Role, L.W. Nicotine enhancement of fast excitatory synaptic transmission in CNS by presynaptic receptors. Science 269, 1692–1696 (1995).
Fowler, C.D. & Kenny, P.J. Nicotine aversion: neurobiological mechanisms and relevance to tobacco dependence vulnerability. Neuropharmacology 76, 533–544 (2014).
Kornetsky, C., Esposito, R.U., McLean, S. & Jacobson, J.O. Intracranial self-stimulation thresholds: a model for the hedonic effects of drugs of abuse. Arch. Gen. Psychiatry 36, 289–292 (1979).
Markou, A. & Koob, G.F. Construct validity of a self-stimulation threshold paradigm: effects of reward and performance manipulations. Physiol. Behav. 51, 111–119 (1992).
Kenny, P.J. Brain reward systems and compulsive drug use. Trends Pharmacol. Sci. 28, 135–141 (2007).
Schaefer, G.J. & Michael, R.P. Task-specific effects of nicotine in rats. Intracranial self-stimulation and locomotor activity. Neuropharmacology 25, 125–131 (1986).
Sartor, C.E. et al. Initial response to cigarettes predicts rate of progression to regular smoking: findings from an offspring-of-twins design. Addict. Behav. 35, 771–778 (2010).
Norton, G.R. & Barske, B. The role of aversion in the rapid-smoking treatment procedure. Addict. Behav. 2, 21–25 (1977).
Merchenthaler, I., Lane, M. & Shughrue, P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J. Comp. Neurol. 403, 261–280 (1999).
Nishikawa, T., Fage, D. & Scatton, B. Evidence for, and nature of, the tonic inhibitory influence of habenulointerpeduncular pathways upon cerebral dopaminergic transmission in the rat. Brain Res. 373, 324–336 (1986).
Fowler, C.D., Lu, Q., Johnson, P.M., Marks, M.J. & Kenny, P.J. Habenular α5 nicotinic receptor subunit signalling controls nicotine intake. Nature 471, 597–601 (2011).
Fowler, C.D., Tuesta, L. & Kenny, P.J. Role of α5* nicotinic acetylcholine receptors in the effects of acute and chronic nicotine treatment on brain reward function in mice. Psychopharmacology (Berl.) http://dx.doi.org/10.1007/s00213-013-3235-1 (2013).
Miranda, M.I. & Bermúdez-Rattoni, F. Reversible inactivation of the nucleus basalis magnocellularis induces disruption of cortical acetylcholine release and acquisition, but not retrieval, of aversive memories. Proc. Natl. Acad. Sci. USA 96, 6478–6482 (1999).
van Bloemendaal, L., Ten Kulve, J.S., la Fleur, S.E., Ijzerman, R.G. & Diamant, M. Effects of glucagon-like peptide 1 on appetite and body weight: focus on the CNS. J. Endocrinol. 221, T1–T16 (2014).
Wang, X.F. et al. Endogenous glucagon-like peptide-1 suppresses high-fat food intake by reducing synaptic drive onto mesolimbic dopamine neurons. Cell Rep. 12, 726–733 (2015).
Scrocchi, L.A. & Drucker, D.J. Effects of aging and a high fat diet on body weight and glucose tolerance in glucagon-like peptide-1 receptor −/− mice. Endocrinology 139, 3127–3132 (1998).
Ayala, J.E. et al. Glucagon-like peptide-1 receptor knockout mice are protected from high-fat diet-induced insulin resistance. Endocrinology 151, 4678–4687 (2010).
Bello, N.T. & Moran, T.H. GLP-1 agonists and satiety. Immunol. Endocr. Metab. Agents Med. Chem. 8, 311–316 (2008).
Gutzwiller, J.P. et al. Glucagon-like peptide-1: a potent regulator of food intake in humans. Gut 44, 81–86 (1999).
Garcia-Diaz, D.E., Jimenez-Montufar, L.L., Guevara-Aguilar, R., Wayner, M.J. & Armstrong, D.L. Olfactory and visceral projections to the nucleus of the solitary tract. Physiol. Behav. 44, 619–624 (1988).
Scott, T.R., Yaxley, S., Sienkiewicz, Z.J. & Rolls, E.T. Gustatory responses in the nucleus tractus solitarius of the alert cynomolgus monkey. J. Neurophysiol. 55, 182–200 (1986).
Hayama, T., Ito, S. & Ogawa, H. Responses of solitary tract nucleus neurons to taste and mechanical stimulations of the oral cavity in decerebrate rats. Exp. Brain Res. 60, 235–242 (1985).
Chang, F.C. & Scott, T.R. Conditioned taste aversions modify neural responses in the rat nucleus tractus solitarius. J. Neurosci. 4, 1850–1862 (1984).
Qin, C., Sun, Y., Chen, J.D. & Foreman, R.D. Gastric electrical stimulation modulates neuronal activity in nucleus tractus solitarii in rats. Auton. Neurosci. 119, 1–8 (2005).
Schwartz, G.J. The role of gastrointestinal vagal afferents in the control of food intake: current prospects. Nutrition 16, 866–873 (2000).
Appleyard, S.M. et al. Visceral afferents directly activate catecholamine neurons in the solitary tract nucleus. J. Neurosci. 27, 13292–13302 (2007).
Mönnikes, H., Lauer, G. & Arnold, R. Peripheral administration of cholecystokinin activates c-fos expression in the locus coeruleus/subcoeruleus nucleus, dorsal vagal complex and paraventricular nucleus via capsaicin-sensitive vagal afferents and CCK-A receptors in the rat. Brain Res. 770, 277–288 (1997).
Willing, A.E. & Berthoud, H.R. Gastric distension-induced c-fos expression in catecholaminergic neurons of rat dorsal vagal complex. Am. J. Physiol. 272, R59–R67 (1997).
Rinaman, L., Baker, E.A., Hoffman, G.E., Stricker, E.M. & Verbalis, J.G. Medullary c-Fos activation in rats after ingestion of a satiating meal. Am. J. Physiol. 275, R262–R268 (1998).
Schmidt, H.D. et al. Glucagon-like peptide-1 receptor activation in the ventral tegmental area decreases the reinforcing efficacy of cocaine. Neuropsychopharmacology 41, 1917–1928 (2016).
Acknowledgements
We thank D.J. Drucker (Lunenfeld Tanenbaum Research Institute, Mount Sinai Hospital, Toronto) for providing the Glp1r knockout mice. This work was supported by the US National Institutes of Health (DA032225 to L.M.T., DK096139 to M.R.H., DA020686 to P.J.K.).
Author information
Authors and Affiliations
Contributions
L.M.T., A.D., Z.C., C.D.F., B.R.L., X.-A.L., Q.L. and M.I. conducted all experiments. T.M.K., M.C., M.P. and M.R.H. provided essential reagents. L.M.T. and P.J.K. designed the experiments, analyzed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
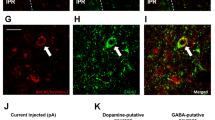
Supplementary Figure 1 Nicotine activates non-catecholaminergic neurons in rostral NTS.
(a) Graphical representation of relative distribution of tyrosine hydroxylase-positive (TH+) neurons in the rostral NTS (rNTS). (b) Representative micrographs of Fos (in red) and TH+ (in green) neurons in the cNTS after nicotine (0.25-1.5 mg/kg) injection. The majority of Fos-positive cells were in non=TH+ cells (identified by white arrows), with few TH+ cells demonstrated Fos immunoreactivity in response to nicotine (identified by yellow arrows).
Supplementary Figure 2 GLP-1 neurons in NTS express α5 nAChR subunits.
40x confocal micrograph of caudal NTS depicting GLP-1-producing neurons (red) and α5* nAChRs (green) from Chrna5-EGFP mice, contrasted by nuclear counterstain (DAPI, blue) (identified by yellow arrows). Yellow insert displays a magnified presentation of the selected cell from the left panel. Note that GFP signal in the caudal NTS is not exclusive to GLP-1 neurons and is detected in neurons that are immunonegative GLP-1 (identified by white arrows). Scale bar: 10μm.
Supplementary Figure 3 Ex-4 dose not alter food responding.
(a) Graphical representation of food responding procedure, showing mouse in operant chamber permitted to respond for food pellets (20 mg). Animals respond on an active lever for food rewards according to a FR5TO20 reinforcement schedule. Each reward earned is delivered into a central food dispenser and triggers the activation of a cue light located above the active lever for 20 sec. Responding on the inactive lever has no scheduled consequence. (b) Mean (± s.e.m.) number of food rewards earned by mice after Ex-4 (10 μg/kg) administration.
Supplementary Figure 4 Nicotine suppresses food responding similarly in wild-type and GLP-1 receptor knockout mice
% Baseline number of food rewards earned by WT (n=4) and GLP-1R KO mice (n=9) after systemic delivery of saline or nicotine. Two-way RM ANOVA, Genotype: F(1, 11)= 0.29, p<0.60; Nicotine: F(1, 11)=19.9, ***p<0.001; Genotype x Nicotine: F(1, 11)=1.4, NS.
Supplementary Figure 5 Cre expression colocalizes almost exclusively with GLP-1 in NTS of Phox2b-Cre mice.
tdTom-expressing neurons in the NTS of the Phox2b::ROSA-tdTom mice were almost exclusively detected in GLP-1+ neurons, but was rarely detected also in TH+ neurons. White arrow highlights a putative TH+, tdTom+ neuron.
Supplementary Figure 6 Chemogenetic activation of GLP-1 neurons does not alter food responding in mice.
Mean (± s.e.m.) number of food rewards earned by Phox2b-Cre mice that had received intra-NTS injection of AAV-FLEX-hM3Dq-mCitrine (n=7) (a), AAV-hM3Dq-mCitrine (n=6) (b) or AAV-EGFP (n=6) (c), or after vehicle (saline) or CNO injection. *P<0.05, paired t-test.
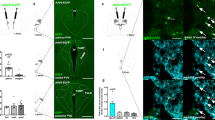
Supplementary Figure 7 Chemogenetic activation of GLP-1 neurons increases activity of IPN neurons.
Representative micrographs showing induction of Fos in IPN of Phox2b-Cre mice that had received intra-NTS injection of AAV-EGFP or AAV-FLEX-hM3Dq-mCitrine following CNO injection. Lower panel shows graphical representation of IPN (gray area) where representative micrographs from upper panels were taken. Scale bar: 100μm.
Supplementary Figure 8 Knockdown of GLP-1 receptors in habenular neurons.
Mean (± s.e.m.) Glp1r expression in MHb relative to GAPDH levels after infusion of AAV-sh-Glp1r-GFP or AAV-GFP viruses into MHb. ***P<0.001; t-test.
Supplementary Figure 9 Knockdown of GLP-1 receptors in habenular neurons does not alter food responding in rats.
Mean (± s.e.m.) number of food rewards earned under an FR5TO20 sec schedule of reinforcement by AAV-GFP and AAV-sh-Glp1r-GFP rats before they had access to nicotine infusions. P>0.1, unpaired t-test; n=6-7 per group.
Supplementary Figure 10 GLP-1 signaling in IPN does not alter food responding.
Mean (± s.e.m.) number of food rewards earned by rats after IPN infusion of vehicle (saline, n=8), Ex-4 (0.1 μg/0.5 μl, n=5) or Ex-9 (20μg/0.5 μl, n=6). Two-way RM ANOVA, treatment: F(2, 48)= 0.05942, p<0.5637; time: F(3, 48)=1.612, p<0.1988; interaction: F(6, 48)=1.731, p<0.1342.
Supplementary Figure 11 Activation of cGMP signaling in IPN does not alter nicotine intake.
Mean (± s.e.m.) number of nicotine infusions earned by rats after IPN infusion of vehicle (saline) or 8-Br-cGMP; n=5. One-way RM ANOVA: F(2, 14)=1.036, p=0.3769.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–11 (PDF 1613 kb)
Rights and permissions
About this article
Cite this article
Tuesta, L., Chen, Z., Duncan, A. et al. GLP-1 acts on habenular avoidance circuits to control nicotine intake. Nat Neurosci 20, 708–716 (2017). https://doi.org/10.1038/nn.4540
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4540
This article is cited by
-
Association of semaglutide with reduced incidence and relapse of cannabis use disorder in real-world populations: a retrospective cohort study
Molecular Psychiatry (2024)
-
Neuroscience in addiction research
Journal of Neural Transmission (2024)
-
Glucagon-like peptide-1 analogues: a new way to quit smoking? (SKIP)—a structured summary of a study protocol for a randomized controlled study
Trials (2023)
-
T cell infiltration into the brain triggers pulmonary dysfunction in murine Cryptococcus-associated IRIS
Nature Communications (2023)
-
Liraglutide attenuates nicotine self-administration as well as nicotine seeking and hyperphagia during withdrawal in male and female rats
Psychopharmacology (2023)