Abstract
Optogenetic interrogation of neural pathways relies on delivery of light-sensitive opsins into tissue and subsequent optical illumination and electrical recording from the regions of interest. Despite the recent development of multifunctional neural probes, integration of these modalities in a single biocompatible platform remains a challenge. We developed a device composed of an optical waveguide, six electrodes and two microfluidic channels produced via fiber drawing. Our probes facilitated injections of viral vectors carrying opsin genes while providing collocated neural recording and optical stimulation. The miniature (<200 μm) footprint and modest weight (<0.5 g) of these probes allowed for multiple implantations into the mouse brain, which enabled opto-electrophysiological investigation of projections from the basolateral amygdala to the medial prefrontal cortex and ventral hippocampus during behavioral experiments. Fabricated solely from polymers and polymer composites, these flexible probes minimized tissue response to achieve chronic multimodal interrogation of brain circuits with high fidelity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Boyden, E.S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268 (2005).
Tye, K.M. & Deisseroth, K. Optogenetic investigation of neural circuits underlying brain disease in animal models. Nat. Rev. Neurosci. 13, 251–266 (2012).
Gradinaru, V., Mogri, M., Thompson, K.R., Henderson, J.M. & Deisseroth, K. Optical deconstruction of parkinsonian neural circuitry. Science 324, 354–359 (2009).
Zhang, F., Aravanis, A.M., Adamantidis, A., de Lecea, L. & Deisseroth, K. Circuit-breakers: optical technologies for probing neural signals and systems. Nat. Rev. Neurosci. 8, 577–581 (2007).
Aravanis, A.M. et al. An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J. Neural Eng. 4, S143–S156 (2007).
Minev, I.R. et al. Biomaterials. Electronic dura mater for long-term multimodal neural interfaces. Science 347, 159–163 (2015).
Park, S.I. et al. Soft, stretchable, fully implantable miniaturized optoelectronic systems for wireless optogenetics. Nat. Biotechnol. 33, 1280–1286 (2015).
Jeong, J.-W. et al. Wireless optofluidic systems for programmable in vivo pharmacology and optogenetics. Cell 162, 662–674 (2015).
Ward, M.P., Rajdev, P., Ellison, C. & Irazoqui, P.P. Toward a comparison of microelectrodes for acute and chronic recordings. Brain Res. 1282, 183–200 (2009).
Polikov, V.S., Tresco, P.A. & Reichert, W.M. Response of brain tissue to chronically implanted neural electrodes. J. Neurosci. Methods 148, 1–18 (2005).
Lind, G., Linsmeier, C.E. & Schouenborg, J. The density difference between tissue and neural probes is a key factor for glial scarring. Sci. Rep. 3, 2942 (2013).
Abouraddy, A.F. et al. Towards multimaterial multifunctional fibres that see, hear, sense and communicate. Nat. Mater. 6, 336–347 (2007).
Tao, G., Stolyarov, A.M. & Abouraddy, A.F. Multimaterial fibers. Int. J. Appl. Glass Sci. 3, 349–368 (2012).
Canales, A. et al. Multifunctional fibers for simultaneous optical, electrical and chemical interrogation of neural circuits in vivo. Nat. Biotechnol. 33, 277–284 (2015).
Guimard, N.K., Gomez, N. & Schmidt, C.E. Conducting polymers in biomedical engineering. Polym. Biomed. Appl. 32, 876–921 (2007).
Green, R.A., Lovell, N.H., Wallace, G.G. & Poole-Warren, L.A. Conducting polymers for neural interfaces: challenges in developing an effective long-term implant. Biomaterials 29, 3393–3399 (2008).
Kuo, J.T.W. et al. Novel flexible Parylene neural probe with 3D sheath structure for enhancing tissue integration. Lab Chip 13, 554–561 (2013).
Kim, B.J. et al. 3D Parylene sheath neural probe for chronic recordings. J. Neural Eng. 10, 045002 (2013).
Huang, J.-C. Carbon black filled conducting polymers and polymer blends. Adv. Polym. Technol. 21, 299–313 (2002).
Roy, N., Sengupta, R. & Bhowmick, A.K. Modifications of carbon for polymer composites and nanocomposites. Prog. Polym. Sci. 37, 781–819 (2012).
Mark, J.E. Polymer Data Handbook (Oxford University Press, 2009).
Bäumer, S. Handbook of Plastic Optics, Second Edition (Wiley, 2011).
Lee, H., Bellamkonda, R.V., Sun, W. & Levenston, M.E. Biomechanical analysis of silicon microelectrode-induced strain in the brain. J. Neural Eng. 2, 81–89 (2005).
Nagel, G. et al. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc. Natl. Acad. Sci. USA 100, 13940–13945 (2003).
Flecknell, P.A. Laboratory Animal Anaesthesia (Elsevier, 2009).
Cardin, J.A. et al. Targeted optogenetic stimulation and recording of neurons in vivo using cell-type-specific expression of Channelrhodopsin-2. Nat. Protoc. 5, 247–254 (2010).
Anikeeva, P. et al. Optetrode: a multichannel readout for optogenetic control in freely moving mice. Nat. Neurosci. 15, 163–170 (2011).
Bacon, S.J., Headlam, A.J.N., Gabbott, P.L.A. & Smith, A.D. Amygdala input to medial prefrontal cortex (mPFC) in the rat: a light and electron microscope study. Brain Res. 720, 211–219 (1996).
Lee, B.R. et al. Maturation of silent synapses in amygdala-accumbens projection contributes to incubation of cocaine craving. Nat. Neurosci. 16, 1644–1651 (2013).
Felix-Ortiz, A.C. et al. BLA to vHPC inputs modulate anxiety-related behaviors. Neuron 79, 658–664 (2013).
Saxena, T. et al. The impact of chronic blood-brain barrier breach on intracortical electrode function. Biomaterials 34, 4703–4713 (2013).
Gunaydin, L.A. et al. Natural neural projection dynamics underlying social behavior. Cell 157, 1535–1551 (2014).
Desai, M. et al. Mapping brain networks in awake mice using combined optical neural control and fMRI. J. Neurophysiol. 105, 1393–1405 (2011).
Farrell, M.S. et al. A Gαs DREADD mouse for selective modulation of cAMP production in striatopallidal neurons. Neuropsychopharmacology 38, 854–862 (2013).
Vardy, E. et al. A new DREADD facilitates the multiplexed chemogenetic interrogation of behavior. Neuron 86, 936–946 (2015).
Nevian, T., Larkum, M.E., Polsky, A. & Schiller, J. Properties of basal dendrites of layer 5 pyramidal neurons: a direct patch-clamp recording study. Nat. Neurosci. 10, 206–214 (2007).
Hunt, R.F., Girskis, K.M., Rubenstein, J.L., Alvarez-Buylla, A. & Baraban, S.C. GABA progenitors grafted into the adult epileptic brain control seizures and abnormal behavior. Nat. Neurosci. 16, 692–697 (2013).
Kravitz, A.V., Tye, L.D. & Kreitzer, A.C. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat. Neurosci. 15, 816–818 (2012).
Paxinos, G. & Franklin, K.B.J. The Mouse Brain in Stereotaxic Coordinates, 2nd Edition (Academic Press, 2004).
Schmitzer-Torbert, N., Jackson, J., Henze, D., Harris, K. & Redish, A.D. Quantitative measures of cluster quality for use in extracellular recordings. Neuroscience 131, 1–11 (2005).
Felix-Ortiz, A.C. & Tye, K.M. Amygdala inputs to the ventral hippocampus bidirectionally modulate social behavior. J. Neurosci. 34, 586–595 (2014).
Jukkola, P., Guerrero, T., Gray, V. & Gu, C. Astrocytes differentially respond to inflammatory autoimmune insults and imbalances of neural activity. Acta Neuropathol. Commun. 1, 70 (2013).
Shi, P. et al. Direct pro-inflammatory effects of prorenin on microglia. PLoS One 9, e92937 (2014).
Wes, P.D. et al. Tau overexpression impacts a neuroinflammation gene expression network perturbed in Alzheimer's disease. PLoS One 9, e106050 (2014).
Siracusa, R. et al. The association of palmitoylethanolamide with luteolin decreases neuroinflammation and stimulates autophagy in Parkinson's disease model. CNS Neurol. Disord. Drug Targets 14, 1350–1365 (2015).
Park, S. et al. Optogenetic control of nerve growth. Sci. Rep. 5, 9669 (2015).
Acknowledgements
The authors are grateful to H. Cho for graphics help during figure preparation. This work was supported in part by the National Institute of Neurological Disorders and Stroke (5R01NS086804, P.A.) National Science Foundation under CAREER award (CBET-1253890, P.A.), Center for Materials Science and Engineering (DMR-1419807, P.A. and Y.F.), Center for Sensorimotor Neural Engineering (EEC-1028725, P.A.) and the McGovern Institute for Brain Research at MIT (P.A. and G.B.C.). S.P. is a recipient of Samsung Scholarship.
Author information
Authors and Affiliations
Contributions
S.P., X.J. and P.A. designed the study. B.G. and X.J. designed the conductive polymer composite gCPE. S.P., Y.G. and X.J. fabricated multifunctional fibers. S.P., Y.G., J.P. and J.K. connectorized fibers to optical, electrical and microfluidic interfaces. S.P., Y.G., A.C., C.L. and J.K. characterized the physical properties of fiber probes. S.P. and Y.G. recorded and analyzed the electrophysiological data. G.B.C. facilitated with design and analysis of behavioral experiments. S.P., C.H.K. and Y.S.Y. conducted behavioral tests. S.P. and R.C. performed immunohistochemistry. Y.F. facilitated with fiber design.All of the authors contributed to the writing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Tuning the diameter of the multifunctional fibers through varying draw speed and stress during the drawing process.
(a) Draw speed tuning over the duration of the process. (b) Diameter of the fiber measured during drawing by the time point. (c) Stress experienced by the fiber during the drawing. (d) Fiber diameter determined by the ratio of feed speed (0.6 mm/min) to the draw speed. Shaded area represents standard deviation. (e) Stability of cross-sectional dimensions for a 10-meter long section of the fiber with 200 μm diameter (the capstan speed was 9.33 m/min). Six different cross-sections spaced by at least 1 m were measured.
Supplementary Figure 2 Mechanical and electrical characterization of the multifunctional fibers.
(a) Bending stiffness measured for a multifunctional fiber, silica optical fiber and a stainless steel microwire. Stiffness was measured by a dynamic mechanical analyzer (DMA, Q800, TA Instrument) in a single cantilever mode with a displacement amplitude of 50 μm. Shaded areas represent standard deviation (n=3). (b) Impedance of gCPE electrodes following multifunctional fiber implantation into the mouse brain. Impedance decreased over the course of the first 3 days of implantation and remained stable over 3 months. Number of samples n=6, * p < 0.05, ** p <0.01, ***p < 0.001 determined by one-way ANOVA. 1 day: p = 0.7972, 2 days: p = 0.0153, 3 days: p = 0.0002, 1 week: p < 0.0001, 2 weeks: p = 0.0005, 1 month: p < 0.0001, 3 months: p = 0.0004, F7, 40 = 8.3. Error bars represent standard deviation. (c) Comparison of commercial CPE and gCPE composite electrodes. SEM images of (i, ii) commercial CPE and (iii, iv) composite graphite-doped gCPE electrodes. Scale bars are (i, iii) 10 μm and (ii, iv) 1 μm, respectively. Composite gCPE electrodes exhibit higher porosity and surface roughness than commercial CPE, which may contribute to the decrease in impedance observed in gCPE electrodes following a soak in a salt solution. (d) Impedance of commercial CPE and gCPE composite electrodes within multifunctional fibers. The impedance was measured when a sinusoidal voltage is 10mV, and Vpp is applied at 1 kHz using a LCR meter (HP4284A, Agilent Technologies). The impedance for commercial CPE electrodes increased proportionally to their length, dominated by sheet-resistance of the material. The impedance of gCPE electrodes does not have proportional correlation with the length likely dominated by the tip geometry. Shaded areas represent standard deviation (Number of samples n=6). (e, f) Evaluation of electrical insulation within fiber probes. (e) An illustration of experimental setup for evaluation of electrical insulation. Impedance of gCPE electrodes was measured for the fibers in surface contact with phosphate buffered saline (PBS) solution and for the fibers immersed into PBS by 1-3 cm. Fiber length was fixed to 5 cm. (f) Impedances of gCPE electrodes in the multifunctional fiber measured for the scenarios in (e) at 1 kHz. No significant differences were identified for the immersed devices as compared to the devices in contact with PBS surface (Number of samples n=6, One-way ANOVA, p = 0.992, F3, 20 = 0.0323. Error bars represent standard deviation. (g) Evaluation of chemical stability of gCPE electrode surfaces in PBS. Cyclic voltammetry (CV) curves collected for a gCPE electrode within a multifunctional fiber at scan rates between 2-100 mV/s. No peaks corresponding to reduction or oxidation are observed within a voltage window exceeding that of extracellular potentials.
Supplementary Figure 3 Optical characterization of the multifunctional fibers.
(a) Optical transmission spectra of multifunctional fibers. The relative transmission is shown for wavelengths within the visible range illustrating the utility of the integrated waveguides for activation of various opsins (ChR2: 473 nm, eNpHR3.0: 589 nm, VChR1: 542 nm, and Chrimson: 627 nm)1,2. (b) Characterization of optical losses for waveguides integrated within multifunctional fiber probes. Optical powers for straight and bent multifunctional fiber-probes were measured at different lengths. Bending angle was 90° and radius curvature was 2.5 mm. The optical losses were calculated to be 1.32 dB/cm and 1.50 dB/cm for straight and bent fiber-probes, respectively. Error bars represent standard deviation (Number of samples n=6). (c, d) Optical losses of integrated polymer waveguides. (c) Infrared thermometry of a fiber probe transmitting 473 nm laser light. The temperature increase of 3.1 °C was observed at steady state (thermodynamic equilibrium) during continuous delivery of 10 mW of 473 nm light, which places upper boundary for simulation energy and duty cycle. For pulsed stimulation at 10 Hz (5 ms pulse width), which was used for experiments in vivo, the temperature increase was 0.8 °C. Scale bar = 500 μm. (d) Transmission losses measured for straight and dent fibers at room temperature, 20 °C and body temperature, 37 °C. Bending angle was 90° and radius curvature was 2.5 mm. No significance was identified between measurements taken at different temperatures. Unpaired student’s t-test: p = 0.6412 for flat fiber, p = 0.5419 for bent fiber. Error bars represent standard deviation, Number of samples n=6. (e, f) Relative optical transmission of waveguides integrated within multifunctional fibers during extreme bending and repeated deformation. (e) Optical transmission for multifunctional fibers measured for repeated bending at 180° with a 2.5 mm radius of curvature (even cycles = straight fiber, odd cycles = bent fiber). Shaded area represents standard deviation (Number of samples n=6). (f) Optical transmission at radii of curvature 0.5, 1, 2.5, 5, 7.5, 10, 12.5, and 15 mm was measured for fibers bent by 90°, 180°, and 270°. Error bars represent standard deviation (Number of samples n=3). (g) Optical transmission losses of the integrated waveguides following multifunctional fiber implantation into the mouse brain. No statistically significant change in transmission loss was observed for devices implanted for up 3 months. Number of samples n=6, One-way ANOVA and Tukey’s comparison test were performed. p = 0.878, F7, 40 = 0.429. Error bars represent standard deviation.
1. Yizhar, O., Fenno, L. E., Davidson, T. J., Mogri, M. & Deisseroth, K. Optogenetics in Neural Systems. Neuron 71, 9–34 (2011).
2. Klapoetke, N. C. et al. Independent optical excitation of distinct neural populations. Nat. Methods 11, 338–46 (2014).
Supplementary Figure 4 Optogenetic interrogation in the mouse mPFC with the multifunctional fiber probes.
(a, b) Expression of AAV5-CamKIIα::ChR2-eYFP in mPFC following delivery through a multifunctional fiber probe. The expression was observed in a wild-type mouse 2 weeks following viral injection. The size of transfected region was defined by the amount of injected virus solution. (a) 0.1 μl and (b) 0.5 μl of virus. Scale bar = 1 mm. (c) Dependence of the amplitude of optically-evoked potentials on the stimulation power density in the mPFC 2 weeks, 1 month, and 3 months following injection of AAV5-CamKIIα::ChR2-eYFP (Number of animals n=8). All electrophysiological recordings, optical stimulation and viral delivery were performed through the multifunctional fiber probes. Error bars represent standard deviation.
Supplementary Figure 5 Electrophysiological recordings and optogenetic stimulation using multifunctional fibers.
(a-f) Acute electrophysiological recording and optogenetic stimulation in the mPFC of transgenic Thy::ChR2-YFP mice. (a-c) Simultaneous optogenetic stimulation (wavelength λ=473 nm, power density 4.3 mW/mm2, 5 ms pulse width, 10 Hz, 1 s stimulation duration, blue markers indicate laser onset) and electrophysiological recording using a multifunctional fiber-probe. (a) Recorded neural activity, (b) raster plot of the detected evoked potentials, and (c) peristimulus time histogram (PSTH) over 20 stimulation trials confirm reproducibility of the response. (d-f) Simultaneous optogenetic stimulation at 100 Hz (other parameters identical to those in (a-c)) and electrophysiological recording with a multifunctional fiber probe. (d) Decaying and uncorrelated response to 100 Hz optical stimulation is consistent with ChR2 activation dynamics3. (e) Raster plot and (f) PSTH over 20 stimulation trials confirm reproducibility of the response. (g-l) Electrophysiological recording and optogenetic stimulation in the mPFC of wild type mice 2 weeks following transfection with AAV5-CaMKIIα::ChR2-eYFP through the multifunctional fiber probe. (a, d) Electrophysiological recording during optogenetic stimulation (λ=473 nm, power density 6.4 mW/mm2, 5 ms pulse width, 10 Hz and 100 Hz (d), 1 s stimulation duration, blue markers indicate laser onset) in the mPFC. (b, e) Raster plots corresponding to 40 trials of recordings in (a, d). (c, f) PSTH over 40 stimulation trials confirms reproducibility of the evoked response. Consistent with ChR2 dynamics optically evoked neural activity is correlated to laser pulses at 10 Hz, but has a decaying and uncorrelated dynamics for 100 Hz stimulation.
3. Cardin, J. a et al. Targeted optogenetic stimulation and recording of neurons in vivo using cell-type-specific expression of Channelrhodopsin-2. Nat. Protoc. 5, 247–254 (2010).
Supplementary Figure 6 Optical stimulation and electrophysiological recordings in the mPFC of mice expressing ChR2-eYFP and eYFP alone.
(a) Neural recordings collected with a fiber probe implanted in the mPFC. The same fiber was used to deliver AAV5-CamKIIα::ChR2-eYFP 14 days prior to the recording. Spontaneous activity is observed prior to the first optical stimulation epoch (10 Hz, 5 ms pulse width, 6.4 mW/mm2). Stimulation epoch is followed by depletion and recovery, which may be attributed to local depletion of neurotransmitters. (b, c) Electrophysiological recordings during optical stimulation in the mPFC using a multifunctional fiber probe (b) 3 days, (c) 2 weeks following transfection with AAV5-CaMKIIα::eYFP. There were no light-evoked signal for both case.
Supplementary Figure 7 Investigation of anxiety level and c-fos expression in the mPFC following optogenetic stimulation with multifunctional fibers.
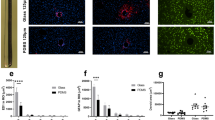
(a, b) Open field test (OFT) for mice transfected with AAV5-CaMKIIα::ChR2-eYFP or AAV5-CaMKIIα::eYFP via multifunctional fibers implanted in the mPFC. Time spent in the center of the open field was measured for mice expressing ChR2-eYFP and eYFP-only controls in the absence of and during (a) 20 Hz and (b) 130 Hz optical stimulation (OFF/ON/OFF cycle, 3 min epoch) through the fiber probes (λ=473 nm, power density 16 mW/mm2, 5 ms pulse width). No statistical significance was found between ChR2-eYFP and eYFP-only mice for both groups (20 Hz OFF_pre: p = 0.505, t = 0.6842, d.f. = 14. 20 Hz ON: p = 0.926, t = -0.0946, d.f. = 14. 20 Hz OFF_post: p = 0.8551, t = 0.186, d.f. = 14. 130 Hz OFF_pre: p = 0.6188, t = -0.5088, d.f. = 14. 130 Hz ON: p = 0.6592, t = 0.4505, d.f. = 14. 130 Hz OFF_post: p = 0.531, t = -0.6425, d.f. = 14). Error bars represent standard deviation (Number of animals n=8). (c-e) C-fos expression in mPFC following optogenetic stimulation with multifunctional fiber probes. (c, d) Representative confocal microscope images of mice transfected with AAV5-CaMKIIα::eYFP or AAV5-CaMKIIα::ChR2-eYFP in mPFC following optical stimulation (λ=473 nm, power density 16 mW/mm2, 20 Hz, 5 ms pulse width). Blue: DAPI, green: eYFP, red: c-fos. (e) The percentage of DAPI-positive cells expressing c-fos in mPFC of ChR2-eYFP transfected mice (86%) was higher than that of eYFP transfected mice (72%). p = 0.0045, t = 3.0243, d.f. = 14. No statistical difference was found in eYFP expression (47% (from DAPI-positive cells) for ChR2-eYFP mice, 45% for eYFP mice, p = 0.3985, t = 0.2619, d.f. = 14). Error bars represent standard deviation (Number of animals n=8).
Supplementary Figure 8 Electrophysiological readout of projection dynamics during optogenetic stimulation in mice implanted with two multifunctional fibers.
(a, b) Electrophysiological recording in the mPFC during optical stimulation using a multifunctional fiber 2 weeks after viral transfection using an identical fiber probe implanted in the BLA. Two types of optically evoked potentials with latencies of 5 ms and 32 ms following laser pulses were observed. The evoked potentials with short latencies are consistent with direct axonal projections from the BLA to mPFC, while the long-latency activity may stem from a multi-synaptic network responses to optical stimulation in the mPFC. (c-e) Dependence of the amplitude of optically-evoked potentials on the stimulation power density 2 weeks, 1 month, and 3 months following injection of AAV5-CamKIIα::ChR2-eYFP using a multifunctional fiber probe implanted in the basolateral amygdala (BLA) (Number of animals n=8). Recordings and optical stimulation (10 Hz, 5 ms pulsewidth) performed in (c) BLA, (d) mPFC, and (e) ventral hippocampus (vHPC). Error bars represent standard deviation.
Supplementary Figure 9 Investigation of locomotion and c-fos expression in vHPC and BLA following optical stimulation of BLA-to-vHPC projection.
(a, b) Locomotor activity in the open field during optical stimulation of inputs from the basolateral amygdala (BLA) into the ventral hippocampus (vHPC). Velocities were measured for mice expressing ChR2-eYFP and eYFP-only controls prior to, during, and after 20 Hz optical stimulation (OFF/ON/OFF cycle, 3 min epoch) (a) in the absence and (b) following injection of 0.1 mM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) solution through the fiber probes (λ=473 nm, power density 16 mW/mm2, 5 ms pulse width). No statistical significance was found between ChR2-eYFP and eYFP-only mice for both groups (No drug_OFF_pre: p = 0.9693, t = 0.0392, d.f. = 14. No drug_ON: p = 0.9323, t = 0.0865, d.f. = 14. No drug_OFF_post: p = 0.7992, t = -0.2593, d.f. = 14. CNQX_ON_pre: p = 0.8688, t = -0.1683, d.f. = 14. CNQX_ON: p = 0.9555, t = 0.0569, d.f. = 14. CNQX_OFF_post: p = 0.9733, t = 0.034, d.f. = 14). Error bars represent standard deviation (Number of animals n=8). (c-h) C-fos expression in vHPC and BLA following optogenetic stimulation of BLA inputs in the vHPC. (c, d) Representative confocal microscope images of the vHPC in mice transfected with AAV5-CaMKIIα::eYFP or AAV5-CaMKIIα::ChR2-eYFP in the BLA following optical stimulation in the vHPC (λ=473 nm, power density 16 mW/mm2, 20 Hz, 5 ms pulse width). Blue: DAPI, green: eYFP, red: c-fos. (e) The percentage of DAPI-positive cells expressing c-fos in the vHPC of ChR2-eYFP transfected mice was significantly higher (63%) than that of eYFP transfected mice (36%). p = 0.0022, t = 3.3863, d.f. = 14. Note, that practically no cell bodies expressing ChR2-eYFP or eYFP were found in vHPC following transfection in the BLA (p = 0.8547, t = -0.2687, d.f. = 14). Error bars represent standard deviation (Number of animals n=8). (f, g) Representative confocal microscope images of BLA in mice transfected with AAV5-CaMKIIα::eYFP or AAV5-CaMKIIα::ChR2-eYFP in this region. Blue: DAPI, green: eYFP, red: c-fos. (h) The percentage of DAPI-positive cells expressing c-fos in the BLA following optical stimulation in the vHPC (identical to (c, d)). No significant difference was found in c-fos expression in the BLA of mice transfected with ChR2-eYFP (52%) or eYFP alone (56%) following stimulation in vHPC (p = 0.3361, t = 0.4321, d.f. = 14). No statistical difference for eYFP expression found as well (p = 0.5391, t = -0.3675, d.f. = 14). Error bars represent standard deviation (Number of animals n=8). Viral delivery and optical stimulation was performed with multifunctional fiber probes implanted in BLA and vHPC, respectively.
Supplementary Figure 10 Calibration of CNQX concentration.
Electrophysiological recording of optically evoked potentials (stimulation parameters: 10 Hz, λ=473 nm, power density 5 mW/mm2, 5 ms pulse width) using multimodal fiber probe implanted in mPFC during and after injection of CNQX solution in PBS at concentrations of (a) 0.01 mM, (b) 0.05 mM, (c) 0.1 mM. R_peak values correspond to maximum amplitudes of optically evoked potentials.
Supplementary Figure 11 Investigation of single-unit potentials within electrophysiological signals recorded with multimodal fibers.
(a-d) Tracking of isolated single neuron (unit) action potentials (spikes) recorded with a with multifunctional fiber probe in mPFC over a period of 12 weeks following implantation in a mouse B. (a) Clusters revealed by principle component analysis (PCA) of isolated action potentials. (b) Average spike waveforms recorded between 1 and 12 weeks corresponding to clusters in (a). (c) Interspike interval (ISI) histograms for isolated neurons 1 and 2 from (a, b). Maximum histogram interval = 1000 ms and bin size = 40 ms. (d) Average firing frequencies for neurons 1 and 2 obtained from ISI histograms. Significant difference confirmed by one way student’s t-test (p < 0.001, t = 13.4275, d.f. = 8). Error bars represent standard deviation (Number of samples n=5). (e-h) Tracking of isolated single neuron (unit) action potentials (spikes) recorded with a with multifunctional fiber probe in mPFC over a period of 12 weeks following implantation in a mouse C. (e) A cluster revealed by principle component analysis (PCA) of isolated action potential. (f) Average spike waveform recorded between 1 and 12 weeks corresponding to the cluster in (e) The shape of average spike waveforms by time point (1 to 12 weeks) from k-mean clustering shown in fig (e). (g) Interspike interval (ISI) histograms for isolated neurons 1 from (e, f). Maximum histogram interval = 1000 ms and bin size = 40 ms. (h) Average firing frequencies obtained from ISI histograms in (g) for the neuron in (f). Error bar represents standard deviation (Number of samples n=5).
Supplementary Figure 12 Illustration of the algorithm used for automated analysis of immunohistochemical data.
Calculation of the area of fluorescently labeled tissue surrounding the implant was performed by a custom algorithm written in MATLAB (Mathworks). First 360 equally distributed radial lines (spaced by 1°) centered in the middle of the hole left by an implant were drawn. For each line, we determined a pixel intensity profile as shown in figure. Points defining the inner (orange stars) and outer boundaries (green rectangles) of the fluorescent area were determined as one standard deviation 1σ above the average fluorescence intensity. All inner and all outer points were connected to each other forming two concentric 720-vertex polygons. Total area was calculated as the difference between the areas of the inner and outer polygons.
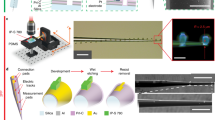
Supplementary Figure 13 Manual connectorization procedure for multifunctional fiber probes.
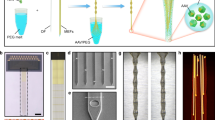
(a) Medical epoxy is used to cover the tip of the fibers to protect its core. Fiber surface is then cleaned by dipping into methanol for 1 min. (b) Dipping into dichloromethane for 1 min is used to dissolve the PC sacrificial layer. (c) The epoxy ends of the fiber are cut off to expose the active elements within the fiber. Fiber is placed under the low magnification microscope. (d-f) Electrode connectorization. (d) The surface of COC and PC insulation is scratched with a scalpel to expose a gCPE electrode. Care should be taken to avoid exposing the neighboring electrodes. (e) A syringe needle is used to apply a small amount of silver paint to the exposed region of the electrode. A different needle can be used to apply a small amount of silver paint thinner to the same region. Silver paint and paint thinner are reapplied up to 3 times until the exposed region is filled with silver paint. A 20 min waiting time is needed ensure complete drying of silver paint. (f) A copper wire (34 gauge) is tightly wrapped over the silver paint. This process can be repeated up to 6 times until the desired number of electrodes are connected to copper wires. For multiple electrodes the exposed regions should be slightly spaced along fiber length to avoid shorting. (g-j) Microfluidic channel connection. (g) Hollow channels within the fiber probes are identified under a microscope. Insulation layer is scratched with a scalpel to expose the channel. It is crucial to confirm that the opening in insulation indeed exposes the channel. Examining propagation of light from the microscope within the cladding may aid in this process. (h) EVA tubing with 0.5 mm inner and 1.5 mm outer diameters is cut to 20 mm length. A hole sufficient for fiber insertion is drilled through the center of the tubing using a needle. (i) The tip of the fiber is fed into a needle and the assembly is gently pushed through the opening in the tubing. The needle is removed. Under the microscope the opening in the fiber cladding is aligned with the tubing. (j) Medical epoxy is mixed and allowed to thicken for 2 min prior to applying it to the outer circumference of the tubing-fiber interface. Sterile water should be used to test the hermetic nature of the microfluidic connection. (k-l) Electrical connector assembly. (k) A female pin connector (Digi-Key) is used for electrodes and a ground wire. The copper wires connected to the electrodes within a fiber are soldered to the pin connector under a microscope. (l) Insulation is removed from both ends of a 2-3 cm long insulated stainless steel wire (34 gauge) using a blade. The ground wire is soldered to a pin on the connector, and the connection is protected with medical epoxy. (m-p) Optical ferrule connection. (m) Optical epoxy is applied to the connector side of the fiber. (n) The fiber is fed into a zirconia ferrule (Thorlabs) such that the fiber tip extends beyond the ferrule length. The optical epoxy is allowed to cure. It is critical to align the optical PC core of the fiber with the center of the ferrule to minimize coupling losses. (o) The tip of the fiber extended past the ferrule length is cut to align with the ferrule edge. The ferrule edge is then polished using a standard fiber polishing kit (Thorlabs). (p) For finer polishing of the fiber tip within the ferrule, the sandpaper within the kit is applied consecutively from large grain (low resolution) to small grain (high resolution).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–13 and Supplementary Tables 1 and 2 (PDF 3213 kb)
41593_2017_BFnn4510_MOESM15_ESM.avi
Open field test performed in mice implanted with multifunctional fiber probes and transfected with AAV5-CaMKIIα::ChR2-eYFP or AAV5-CaMKIIα::eYFP in the mPFC during 20 Hz optical stimulation. (AVI 16099 kb)
41593_2017_BFnn4510_MOESM16_ESM.avi
Open field test performed in mice implanted with multifunctional fiber probes and transfected with AAV5-CaMKIIα::ChR2-eYFP or AAV5-CaMKIIα::eYFP in the mPFC during 130 Hz optical stimulation. (AVI 15863 kb)
41593_2017_BFnn4510_MOESM17_ESM.avi
Open field exploration during 20 Hz optical stimulation in the vHPC in mice implanted with multifunctional fiber probes in vHPC and BLA, and transfected with AAV5-CaMKIIα::ChR2-eYFP or AAV5-CaMKIIα::eYFP in the BLA. No drug was injected to the vHPC. (AVI 15814 kb)
41593_2017_BFnn4510_MOESM18_ESM.avi
Open field exploration during 20 Hz optical stimulation in the vHPC in mice implanted with multifunctional fiber probes in the vHPC and BLA, and transfected with AAV5-CaMKIIα::ChR2-eYFP or AAV5-CaMKIIα::eYFP in the BLA. 0.1 mM of CNQX solution was infused into the vHPC 10 min prior to the behavioral test. (AVI 16097 kb)
Rights and permissions
About this article
Cite this article
Park, S., Guo, Y., Jia, X. et al. One-step optogenetics with multifunctional flexible polymer fibers. Nat Neurosci 20, 612–619 (2017). https://doi.org/10.1038/nn.4510
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4510
This article is cited by
-
Self-assembled ultraflexible probes for long-term neural recordings and neuromodulation
Nature Protocols (2023)
-
Conductance stable and mechanically durable bi-layer EGaIn composite-coated stretchable fiber for 1D bioelectronics
Nature Communications (2023)
-
Recent developments in multifunctional neural probes for simultaneous neural recording and modulation
Microsystems & Nanoengineering (2023)
-
Polydimethylsiloxane as a more biocompatible alternative to glass in optogenetics
Scientific Reports (2023)
-
A mosquito mouthpart-like bionic neural probe
Microsystems & Nanoengineering (2023)