Abstract
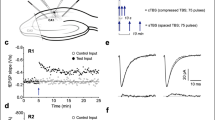
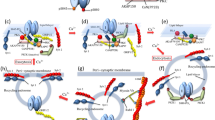
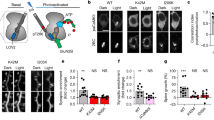
Long-term potentiation (LTP) in the rat hippocampus is the most extensively studied cellular model for learning and memory. Induction of classical LTP involves an NMDA-receptor- and calcium-dependent increase in functional synaptic AMPA receptors, mediated by enhanced recycling of internalized AMPA receptors back to the postsynaptic membrane. Here we report a physiologically relevant NMDA-receptor-independent mechanism that drives increased AMPA receptor recycling and LTP. This pathway requires the metabotropic action of kainate receptors and activation of G protein, protein kinase C and phospholipase C. Like classical LTP, kainate-receptor-dependent LTP recruits recycling endosomes to spines, enhances synaptic recycling of AMPA receptors to increase their surface expression and elicits structural changes in spines, including increased growth and maturation. These data reveal a new and, to our knowledge, previously unsuspected role for postsynaptic kainate receptors in the induction of functional and structural plasticity in the hippocampus.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Change history
08 March 2017
In the version of this article initially published online, the graph in the second row of Figure 5c was a duplicate of the one in the first row. Also, "EPSC amplitudes" in the legend to Figure 5d should have read "EPSP slopes." Finally, the units on the x axes in Figure 4c, 5c, 6f and 7d should have been μm, not mm. The errors have been corrected in the print, PDF and HTML versions of this article.
References
Malenka, R.C. & Bear, M.F. LTP and LTD: an embarrassment of riches. Neuron 44, 5–21 (2004).
Lu, W. et al. Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron 29, 243–254 (2001).
Park, M., Penick, E.C., Edwards, J.G., Kauer, J.A. & Ehlers, M.D. Recycling endosomes supply AMPA receptors for LTP. Science 305, 1972–1975 (2004).
Park, M. et al. Plasticity-induced growth of dendritic spines by exocytic trafficking from recycling endosomes. Neuron 52, 817–830 (2006).
Contractor, A., Mulle, C. & Swanson, G.T. Kainate receptors coming of age: milestones of two decades of research. Trends Neurosci. 34, 154–163 (2011).
Lerma, J. & Marques, J.M. Kainate receptors in health and disease. Neuron 80, 292–311 (2013).
González-González, I.M. et al. Kainate receptor trafficking. WIRES Membrane Trasnsport and Signalling 1, 31–44 (2012).
Rodríguez-Moreno, A. & Lerma, J. Kainate receptor modulation of GABA release involves a metabotropic function. Neuron 20, 1211–1218 (1998).
Rozas, J.L., Paternain, A.V. & Lerma, J. Noncanonical signaling by ionotropic kainate receptors. Neuron 39, 543–553 (2003).
Rutkowska-Wlodarczyk, I. et al. A proteomic analysis reveals the interaction of GluK1 ionotropic kainate receptor subunits with Go proteins. J. Neurosci. 35, 5171–5179 (2015).
Melyan, Z., Wheal, H.V. & Lancaster, B. Metabotropic-mediated kainate receptor regulation of IsAHP and excitability in pyramidal cells. Neuron 34, 107–114 (2002).
Fisahn, A., Heinemann, S. & McBain, C.J. The kainate receptor subunit GluR6 mediates metabotropic regulation of the slow and medium AHP currents in mouse hippocampal neurons. J. Physiol. 562, 199–203 (2004).
Melyan, Z., Lancaster, B. & Wheal, H.V. Metabotropic regulation of intrinsic excitability by synaptic activation of kainate receptors. J. Neurosci. 24, 4530–4534 (2004).
Ruiz, A., Sachidhanandam, S., Utvik, J.K., Coussen, F. & Mulle, C. Distinct subunits in heteromeric kainate receptors mediate ionotropic and metabotropic function at hippocampal mossy fiber synapses. J. Neurosci. 25, 11710–11718 (2005).
Rivera, R., Rozas, J.L. & Lerma, J. PKC-dependent autoregulation of membrane kainate receptors. EMBO J. 26, 4359–4367 (2007).
Martin, S., Bouschet, T., Jenkins, E.L., Nishimune, A. & Henley, J.M. Bidirectional regulation of kainate receptor surface expression in hippocampal neurons. J. Biol. Chem. 283, 36435–36440 (2008).
Selak, S. et al. A role for SNAP25 in internalization of kainate receptors and synaptic plasticity. Neuron 63, 357–371 (2009).
Carta, M. et al. CaMKII-dependent phosphorylation of GluK5 mediates plasticity of kainate receptors. EMBO J. 32, 496–510 (2013).
González-González, I.M. & Henley, J.M. Postsynaptic kainate receptor recycling and surface expression are regulated by metabotropic autoreceptor signalling. Traffic 14, 810–822 (2013).
Bureau, I., Bischoff, S., Heinemann, S.F. & Mulle, C. Kainate receptor-mediated responses in the CA1 field of wild-type and GluR6-deficient mice. J. Neurosci. 19, 653–663 (1999).
Grover, L.M. & Teyler, T.J. N-methyl-D-aspartate receptor-independent long-term potentiation in area CA1 of rat hippocampus: input-specific induction and preclusion in a non-tetanized pathway. Neuroscience 49, 7–11 (1992).
Grover, L.M. & Teyler, T.J. Two components of long-term potentiation induced by different patterns of afferent activation. Nature 347, 477–479 (1990).
Behrens, C.J., van den Boom, L.P., de Hoz, L., Friedman, A. & Heinemann, U. Induction of sharp wave-ripple complexes in vitro and reorganization of hippocampal networks. Nat. Neurosci. 8, 1560–1567 (2005).
Grover, L.M. Evidence for postsynaptic induction and expression of NMDA receptor independent LTP. J. Neurophysiol. 79, 1167–1182 (1998).
Huang, Y.Y. & Malenka, R.C. Examination of TEA-induced synaptic enhancement in area CA1 of the hippocampus: the role of voltage-dependent Ca2+ channels in the induction of LTP. J. Neurosci. 13, 568–576 (1993).
Brown, T.C., Correia, S.S., Petrok, C.N. & Esteban, J.A. Functional compartmentalization of endosomal trafficking for the synaptic delivery of AMPA receptors during long-term potentiation. J. Neurosci. 27, 13311–13315 (2007).
van Weert, A.W., Geuze, H.J., Groothuis, B. & Stoorvogel, W. Primaquine interferes with membrane recycling from endosomes to the plasma membrane through a direct interaction with endosomes which does not involve neutralisation of endosomal pH nor osmotic swelling of endosomes. Eur. J. Cell Biol. 79, 394–399 (2000).
Mollenhauer, H.H., James Morré, D. & Rowe, L.D. Alteration of intracellular traffic by monensin; mechanism, specificity and relationship to toxicity. Biochim Biophys Acta 1031, 225–246 (1990).
Sihra, T.S., Flores, G. & Rodriguez-Moreno, A. Kainate receptors: multiple roles in neuronal plasticity. Neuroscientist 20, 29–43 (2014).
Lynch, G., Larson, J., Kelso, S., Barrionuevo, G. & Schottler, F. Intracellular injections of EGTA block induction of hippocampal long-term potentiation. Nature 305, 719–721 (1983).
Malenka, R.C., Kauer, J.A., Zucker, R.S. & Nicoll, R.A. Postsynaptic calcium is sufficient for potentiation of hippocampal synaptic transmission. Science 242, 81–84 (1988).
Zong, X. & Lux, H.D. Augmentation of calcium channel currents in response to G protein activation by GTP gamma S in chick sensory neurons. J. Neurosci. 14, 4847–4853 (1994).
Pinheiro, P.S. et al. Selective block of postsynaptic kainate receptors reveals their function at hippocampal mossy fiber synapses. Cereb. Cortex 23, 323–331 (2013).
Marques, J.M. et al. CRMP2 tethers kainate receptor activity to cytoskeleton dynamics during neuronal maturation. J. Neurosci. 33, 18298–18310 (2013).
Lanore, F. et al. Deficits in morphofunctional maturation of hippocampal mossy fiber synapses in a mouse model of intellectual disability. J. Neurosci. 32, 17882–17893 (2012).
Tashiro, A., Dunaevsky, A., Blazeski, R., Mason, C.A. & Yuste, R. Bidirectional regulation of hippocampal mossy fiber filopodial motility by kainate receptors: a two-step model of synaptogenesis. Neuron 38, 773–784 (2003).
Engert, F. & Bonhoeffer, T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature 399, 66–70 (1999).
Matsuzaki, M. et al. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat. Neurosci. 4, 1086–1092 (2001).
Matsuzaki, M. Factors critical for the plasticity of dendritic spines and memory storage. Neurosci. Res. 57, 1–9 (2007).
Wang, Z. et al. Myosin Vb mobilizes recycling endosomes and AMPA receptors for postsynaptic plasticity. Cell 135, 535–548 (2008).
Lerma, J. Kainate receptor physiology. Curr. Opin. Pharmacol. 6, 89–97 (2006).
Malenka, R.C. & Nicoll, R.A. Long-term potentiation--a decade of progress? Science 285, 1870–1874 (1999).
Ylinen, A. et al. Sharp wave-associated high-frequency oscillation (200 Hz) in the intact hippocampus: network and intracellular mechanisms. J. Neurosci. 15, 30–46 (1995).
O'Neill, J., Senior, T. & Csicsvari, J. Place-selective firing of CA1 pyramidal cells during sharp wave/ripple network patterns in exploratory behavior. Neuron 49, 143–155 (2006).
Ego-Stengel, V. & Wilson, M.A. Disruption of ripple-associated hippocampal activity during rest impairs spatial learning in the rat. Hippocampus 20, 1–10 (2010).
Kato, H.K., Kassai, H., Watabe, A.M., Aiba, A. & Manabe, T. Functional coupling of the metabotropic glutamate receptor, InsP3 receptor and L-type Ca2+ channel in mouse CA1 pyramidal cells. J. Physiol. (Lond.) 590, 3019–3034 (2012).
Miyazaki, K. & Ross, W.N. Ca2+ sparks and puffs are generated and interact in rat hippocampal CA1 pyramidal neuron dendrites. J. Neurosci. 33, 17777–17788 (2013).
Crépel, V. & Mulle, C. Physiopathology of kainate receptors in epilepsy. Curr. Opin. Pharmacol. 20, 83–88 (2015).
Motazacker, M.M. et al. A defect in the ionotropic glutamate receptor 6 gene (GRIK2) is associated with autosomal recessive mental retardation. Am. J. Hum. Genet. 81, 792–798 (2007).
Petrovic, M.M. et al. Inhibition of post-synaptic Kv7/KCNQ/M channels facilitates long-term potentiation in the hippocampus. PLoS One 7, e30402 (2012).
Bezanilla, F. & Armstrong, C.M. Inactivation of the sodium channel. I. Sodium current experiments. J. Gen. Physiol. 70, 549–566 (1977).
Cottrell, J.R., Borok, E., Horvath, T.L. & Nedivi, E. CPG2: a brain- and synapse-specific protein that regulates the endocytosis of glutamate receptors. Neuron 44, 677–690 (2004).
Zürner, M., Mittelstaedt, T., tom Dieck, S., Becker, A. & Schoch, S. Analyses of the spatiotemporal expression and subcellular localization of liprin-α proteins. J. Comp. Neurol. 519, 3019–3039 (2011).
King, A.E., Chung, R.S., Vickers, J.C. & Dickson, T.C. Localization of glutamate receptors in developing cortical neurons in culture and relationship to susceptibility to excitotoxicity. J. Comp. Neurol. 498, 277–294 (2006).
Akchiche, N. et al. Differentiation and neural integration of hippocampal neuronal progenitors: signaling pathways sequentially involved. Hippocampus 20, 949–961 (2010).
Bombeiro, A.L. et al. Correction: MHC-I and PirB upregulation in the central and peripheral nervous system following sciatic nerve injury. PLoS One 11, e0165185 (2016).
Unal, G., Joshi, A., Viney, T.J., Kis, V. & Somogyi, P. Synaptic targets of medial septal projections in the hippocampus and extrahippocampal cortices of the mouse. J. Neurosci. 35, 15812–15826 (2015).
Torigoe, M., Yamauchi, K., Zhu, Y., Kobayashi, H. & Murakami, F. Association of astrocytes with neurons and astrocytes derived from distinct progenitor domains in the subpallium. Sci. Rep. 5, 12258 (2015).
Grelli, K.N. et al. Alteration of isocitrate dehydrogenase following acute ischemic injury as a means to improve cellular energetic status in neuroadaptation. CNS Neurol. Disord. Drug Targets 12, 849–860 (2013).
Rodriguez, A., Ehlenberger, D.B., Hof, P.R. & Wearne, S.L. Rayburst sampling, an algorithm for automated three-dimensional shape analysis from laser scanning microscopy images. Nat. Protoc. 1, 2152–2161 (2006).
Rodriguez, A., Ehlenberger, D.B., Dickstein, D.L., Hof, P.R. & Wearne, S.L. Automated three-dimensional detection and shape classification of dendritic spines from fluorescence microscopy images. PLoS One 3, e1997 (2008).
Bodon, G. et al. Charged multivesicular body protein 2B (CHMP2B) of the endosomal sorting complex required for transport-III (ESCRT-III) polymerizes into helical structures deforming the plasma membrane. J. Biol. Chem. 286, 40276–40286 (2011).
Chamberlain, S.E. et al. SUMOylation and phosphorylation of GluK2 regulate kainate receptor trafficking and synaptic plasticity. Nat. Neurosci. 15, 845–852 (2012).
Gerges, N.Z., Brown, T.C., Correia, S.S. & Esteban, J.A. Analysis of Rab protein function in neurotransmitter receptor trafficking at hippocampal synapses. Methods Enzymol. 403, 153–166 (2005).
Acknowledgements
We are grateful to P. Rubin and N. Grosjean for excellent technical support, A. Singh for his help in some follow-up experiments, M. Gajic for the advice about statistics and J. Esteban (CBMSO, Madrid) for providing Rab constructs. We are grateful for financial support from the ERC (Proposal no. 232881), MRC (MR/L003791), BHF (PG/14/60/31014) and BBSRC (BB/K014366 and BB/K014358) to J.M.H.; EMBO Fellowships to I.M.G.-G. (ALTF 224-2009 and ASTF 438-2011) and M.M.P. (ASTF 232-2011); a grant from MRC (MR/M023729/1) to M.M.P.; grants from the Centre National de la Recherche Scientifique, the Conseil Régional d'Aquitaine, the Labex BRAIN and the Fundacao para a Ciencia e a Tecnologia to C.M. and S.V.d.S.; support from the Czech Science Foundation (GACR): 17-02300S) and Research Project of the AS CR RVO (67985823) to L.V.; and a grant from the Department of Science and Technology (DST) – Young Scientist Scheme (SERB/LS-779/2013) to J.P.C.
Author information
Authors and Affiliations
Contributions
I.M.G.-G. designed and performed the biochemistry and imaging experiments and participated in electrophysiological experiments; M.M.P. designed and performed agonist- and stimulation-evoked electrophysiology and participated in imaging experiments. S.V.d.S. performed electrophysiology in wild-type and mice hippocampal slices; C.M. provided knockout mice and extensive advice; J.P.C. performed the MK-801, D-APV and CNQX dual pathway electrophysiological experiments. L.V. provided facilities and reagents and helped analyses the electrophysiological data. J.M.H. instigated the study and provided overall supervision and management. J.M.H., M.I.G.-G. and M.M.P. designed the study, analyzed the data and wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–15 (PDF 3288 kb)
Supplementary Table 1
Supplementary statistical information (XLSX 23 kb)
Rights and permissions
About this article
Cite this article
Petrovic, M., Viana da Silva, S., Clement, J. et al. Metabotropic action of postsynaptic kainate receptors triggers hippocampal long-term potentiation. Nat Neurosci 20, 529–539 (2017). https://doi.org/10.1038/nn.4505
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4505
This article is cited by
-
Electroacupuncture ameliorates learning and memory deficits via hippocampal 5-HT1A receptors and the PKA signaling pathway in rats with ischemic stroke
Metabolic Brain Disease (2020)
-
Damaging coding variants within kainate receptor channel genes are enriched in individuals with schizophrenia, autism and intellectual disabilities
Scientific Reports (2019)
-
Exciting Times: New Advances Towards Understanding the Regulation and Roles of Kainate Receptors
Neurochemical Research (2019)
-
Development of Cortical Pyramidal Cell and Interneuronal Dendrites: a Role for Kainate Receptor Subunits and NETO1
Molecular Neurobiology (2019)
-
Kainate receptors can LTP
Nature Reviews Neuroscience (2017)