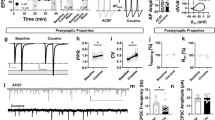
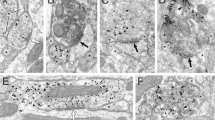
Abstract
Afferent inputs to the ventral tegmental area (VTA) control reward-related behaviors through regulation of dopamine neuron activity. The nucleus accumbens (NAc) provides one of the most prominent projections to the VTA; however, recent studies have provided conflicting evidence regarding the function of these inhibitory inputs. Using optogenetics, cell-specific ablation, whole cell patch-clamp and immuno-electron microscopy, we found that NAc inputs synapsed directly onto dopamine neurons, preferentially activating GABAB receptors. GABAergic inputs from the NAc and local VTA GABA neurons were differentially modulated and activated separate receptor populations in dopamine neurons. Genetic deletion of GABAB receptors from dopamine neurons in adult mice did not affect general or morphine-induced locomotor activity, but markedly increased cocaine-induced locomotion. Collectively, our findings demonstrate notable selectivity in the inhibitory architecture of the VTA and suggest that long-range GABAergic inputs to dopamine neurons fundamentally regulate behavioral responses to cocaine.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Accession codes
Change history
27 March 2017
In the version of this article initially published, the y-axis scale in Figure 4c was labeled 0–150 instead of 0–300, the gray data points in Figure 6g were duplicates of the black data points in Figure 6f, and the error bars were missing from the green trace in Figure 7e. The errors have been corrected in the HTML and PDF versions of the article.
References
Stuber, G.D. et al. Reward-predictive cues enhance excitatory synaptic strength onto midbrain dopamine neurons. Science 321, 1690–1692 (2008).
Di Chiara, G. & Imperato, A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc. Natl. Acad. Sci. USA 85, 5274–5278 (1988).
Ribak, C.E., Vaughn, J.E., Saito, K., Barber, R. & Roberts, E. Immunocytochemical localization of glutamate decarboxylase in rat substantia nigra. Brain Res. 116, 287–298 (1976).
Bolam, J.P. & Smith, Y. The GABA and substance P input to dopaminergic neurones in the substantia nigra of the rat. Brain Res. 529, 57–78 (1990).
Henny, P. et al. Structural correlates of heterogeneous in vivo activity of midbrain dopaminergic neurons. Nat. Neurosci. 15, 613–619 (2012).
Bayer, V.E. & Pickel, V.M. GABA-labeled terminals form proportionally more synapses with dopaminergic neurons containing low densities of tyrosine hydroxylase-immunoreactivity in rat ventral tegmental area. Brain Res. 559, 44–55 (1991).
Johnson, S.W. & North, R.A. Two types of neurone in the rat ventral tegmental area and their synaptic inputs. J. Physiol. (Lond.) 450, 455–468 (1992).
Sugita, S., Johnson, S.W. & North, R.A. Synaptic inputs to GABAA and GABAB receptors originate from discrete afferent neurons. Neurosci. Lett. 134, 207–211 (1992).
Cameron, D.L. & Williams, J.T. Dopamine D1 receptors facilitate transmitter release. Nature 366, 344–347 (1993).
Cameron, D.L. & Williams, J.T. Cocaine inhibits GABA release in the VTA through endogenous 5-HT. J. Neurosci. 14, 6763–6767 (1994).
Watabe-Uchida, M., Zhu, L., Ogawa, S.K., Vamanrao, A. & Uchida, N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron 74, 858–873 (2012).
Menegas, W. et al. Dopamine neurons projecting to the posterior striatum form an anatomically distinct subclass. eLife 4, e10032 (2015).
Xia, Y. et al. Nucleus accumbens medium spiny neurons target non-dopaminergic neurons in the ventral tegmental area. J. Neurosci. 31, 7811–7816 (2011).
Bocklisch, C. et al. Cocaine disinhibits dopamine neurons by potentiation of GABA transmission in the ventral tegmental area. Science 341, 1521–1525 (2013).
Johnson, S.W. & North, R.A. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J. Neurosci. 12, 483–488 (1992).
Einhorn, L.C., Johansen, P.A. & White, F.J. Electrophysiological effects of cocaine in the mesoaccumbens dopamine system: studies in the ventral tegmental area. J. Neurosci. 8, 100–112 (1988).
Somogyi, P., Bolam, J.P., Totterdell, S. & Smith, A.D. Monosynaptic input from the nucleus accumbens–ventral striatum region to retrogradely labelled nigrostriatal neurones. Brain Res. 217, 245–263 (1981).
Matsui, A., Jarvie, B.C., Robinson, B.G., Hentges, S.T. & Williams, J.T. Separate GABA afferents to dopamine neurons mediate acute action of opioids, development of tolerance, and expression of withdrawal. Neuron 82, 1346–1356 (2014).
Gerfen, C.R. et al. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 250, 1429–1432 (1990).
Al-Hasani, R. et al. Distinct subpopulations of nucleus accumbens dynorphin neurons drive aversion and reward. Neuron 87, 1063–1077 (2015).
Krashes, M.J. et al. An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature 507, 238–242 (2014).
Chavkin, C., James, I.F. & Goldstein, A. Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science 215, 413–415 (1982).
Lu, X.-Y., Ghasemzadeh, M.B. & Kalivas, P.W. Regional distribution and cellular localization of γ-aminobutyric acid subtype 1 receptor mRNA in the rat brain. J. Comp. Neurol. 407, 166–182 (1999).
Chieng, B., Azriel, Y., Mohammadi, S. & Christie, M.J. Distinct cellular properties of identified dopaminergic and GABAergic neurons in the mouse ventral tegmental area. J. Physiol. (Lond.) 589, 3775–3787 (2011).
Yamaguchi, T., Sheen, W. & Morales, M. Glutamatergic neurons are present in the rat ventral tegmental area. Eur. J. Neurosci. 25, 106–118 (2007).
Wanat, M.J., Hopf, F.W., Stuber, G.D., Phillips, P.E.M. & Bonci, A. Corticotropin-releasing factor increases mouse ventral tegmental area dopamine neuron firing through a protein kinase C-dependent enhancement of Ih. J. Physiol. (Lond.) 586, 2157–2170 (2008).
Isaacson, J.S., Solís, J.M. & Nicoll, R.A. Local and diffuse synaptic actions of GABA in the hippocampus. Neuron 10, 165–175 (1993).
Scanziani, M. GABA spillover activates postsynaptic GABA(B) receptors to control rhythmic hippocampal activity. Neuron 25, 673–681 (2000).
Boyes, J. & Bolam, J.P. The subcellular localization of GABA(B) receptor subunits in the rat substantia nigra. Eur. J. Neurosci. 18, 3279–3293 (2003).
Min, M.-Y., Rusakov, D.A. & Kullmann, D.M. Activation of AMPA, kainate, and metabotropic receptors at hippocampal mossy fiber synapses: role of glutamate diffusion. Neuron 21, 561–570 (1998).
Ford, C.P., Gantz, S.C., Phillips, P.E.M. & Williams, J.T. Control of extracellular dopamine at dendrite and axon terminals. J. Neurosci. 30, 6975–6983 (2010).
van Zessen, R., Phillips, J.L., Budygin, E.A. & Stuber, G.D. Activation of VTA GABA neurons disrupts reward consumption. Neuron 73, 1184–1194 (2012).
Tan, K.R. et al. GABA neurons of the VTA drive conditioned place aversion. Neuron 73, 1173–1183 (2012).
Kaupmann, K. et al. GABA(B)-receptor subtypes assemble into functional heteromeric complexes. Nature 396, 683–687 (1998).
Gompf, H.S., Budygin, E.A., Fuller, P.M. & Bass, C.E. Targeted genetic manipulations of neuronal subtypes using promoter-specific combinatorial AAVs in wild-type animals. Front. Behav. Neurosci. 9, 152 (2015).
Haller, C. et al. Floxed allele for conditional inactivation of the GABAB(1) gene. Genesis 40, 125–130 (2004).
Zombeck, J.A., Swearingen, S.P. & Rhodes, J.S. Acute locomotor responses to cocaine in adolescents vs. adults from four divergent inbred mouse strains. Genes Brain Behav. 9, 892–898 (2010).
Eisener-Dorman, A.F., Grabowski-Boase, L. & Tarantino, L.M. Cocaine locomotor activation, sensitization and place preference in six inbred strains of mice. Behav. Brain Funct. 7, 29 (2011).
Margolis, E.B., Toy, B., Himmels, P., Morales, M. & Fields, H.L. Identification of rat ventral tegmental area GABAergic neurons. PLoS One 7, e42365 (2012).
Labouèbe, G. et al. RGS2 modulates coupling between GABAB receptors and GIRK channels in dopamine neurons of the ventral tegmental area. Nat. Neurosci. 10, 1559–1568 (2007).
Isaacson, J.S. & Scanziani, M. How inhibition shapes cortical activity. Neuron 72, 231–243 (2011).
Oláh, S. et al. Regulation of cortical microcircuits by unitary GABA-mediated volume transmission. Nature 461, 1278–1281 (2009).
Freund, T.F. & Katona, I. Perisomatic inhibition. Neuron 56, 33–42 (2007).
Lovett-Barron, M. et al. Regulation of neuronal input transformations by tunable dendritic inhibition. Nat. Neurosci. 15, 423–430, S1–S3 (2012).
Kalivas, P.W., Duffy, P. & Eberhardt, H. Modulation of A10 dopamine neurons by gamma-aminobutyric acid agonists. J. Pharmacol. Exp. Ther. 253, 858–866 (1990).
Grace, A.A. & Bunney, B.S. Paradoxical GABA excitation of nigral dopaminergic cells: indirect mediation through reticulata inhibitory neurons. Eur. J. Pharmacol. 59, 211–218 (1979).
Jacobson, L.H. et al. Differential roles of GABAB1 subunit isoforms on locomotor responses to acute and repeated administration of cocaine. Behav. Brain Res. 298 Pt B: 12–16 (2016).
Ling, W., Shoptaw, S. & Majewska, D. Baclofen as a cocaine anti-craving medication: a preliminary clinical study. Neuropsychopharmacology 18, 403–404 (1998).
Slattery, D.A., Markou, A., Froestl, W. & Cryan, J.F. The GABAB receptor-positive modulator GS39783 and the GABAB receptor agonist baclofen attenuate the reward-facilitating effects of cocaine: intracranial self-stimulation studies in the rat. Neuropsychopharmacology 30, 2065–2072 (2005).
Parker, J.G. et al. Attenuating GABA(A) receptor signaling in dopamine neurons selectively enhances reward learning and alters risk preference in mice. J. Neurosci. 31, 17103–17112 (2011).
Vong, L. et al. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron 71, 142–154 (2011).
Gong, S. et al. Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J. Neurosci. 27, 9817–9823 (2007).
Ting, J.T., Daigle, T.L., Chen, Q. & Feng, G. Acute brain slice methods for adult and aging animals: application of targeted patch clamp analysis and optogenetics. Methods Mol. Biol. 1183, 221–242 (2014).
Zucker, R.S. & Regehr, W.G. Short-term synaptic plasticity. Annu. Rev. Physiol. 64, 355–405 (2002).
Zhang, S. et al. Dopaminergic and glutamatergic microdomains in a subset of rodent mesoaccumbens axons. Nat. Neurosci. 18, 386–392 (2015).
McDevitt, R.A. et al. Serotonergic versus nonserotonergic dorsal raphe projection neurons: differential participation in reward circuitry. Cell Reports 8, 1857–1869 (2014).
Britt, J.P. et al. Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron 76, 790–803 (2012).
Acknowledgements
We thank members of the Bonci lab for insightful discussions and careful reading of the manuscript. We thank the NIDA Histology Core for help with in situ hybridization experiments, B. Sadacca for help with statistical analysis, K. Deisseroth (Stanford University) for the generation of optogenetic constructs, and B. Lowell (Beth Israel Deaconess Medical Center) for Dyn-Cre and VGAT-Cre transgenic mice. This work was supported by the Intramural Research Program at the National Institute on Drug Abuse.
Author information
Authors and Affiliations
Contributions
N.J.E., A.B., H.A.T., R.A.M., M.M.and M.P. designed the experiments. N.J.E. performed electrophysiological and behavioral experiments. J.W. determined virus localizations and S.Z. performed electron microscopy experiments. B.B. provided critical reagents. N.J.E. analyzed the data. N.J.E. and A.B. wrote the paper with contributions from all of the other authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Localization of virus injections.
(a) Schematic showing the approximate center of AAV-DIO-ChR2-YFP injection sites in the NAc of Dyn-cre mice (n=8 mice, 16 injections; caudate/putamen, CPu; nucleus accumbens core, NAcC; nucleus accumbens shell, NAcSh). (b) Representative GABAB oIPSCs at different stimution frequencies.
Supplementary Figure 2 D2 MSNs project to the ventral pallidum, but not directly to the VTA.
(a) Schematic of experimental protocol. Cre-dependent ChR2-YFP was injected into the VTA of A2A-cre mice and whole-cell patch-clamp recordings were performed in VTA dopamine and GABA neurons and in ventral pallidal neurons. (b) Summary data of light-evoked GABAA oIPSCs in voltage clamp recordings (Vm=-70 mV) of VTA dopamine (blue), VTA GABA (red), and ventral pallidal neurons (purple; n=10, 8, and 11 cells; one-way ANOVA, F(28)=8.606, p<0.01). (c) Representative traces of light-evoked GABAA IPSCs in VTA dopamine (blue), VTA GABA (red) and ventral pallidal neurons (purple). Data are shown as mean ± s.e.m.
Supplementary Figure 3 GABABRs are strongly expressed in dopamine neurons throughout the midbrain.
(a) Wide-field image showing TH immunoreactivity (brown) and GABAB1 in situ (black; scale bar, 300 μm). (b) TH neurons co-express GABAB1 mRNA (scale bar, 25 μm). (c) Percentage of TH neurons co-expressing GABAB1 mRNA in the substantia nigra pars compacta (SNc) and VTA (n=3 mice, 4 sections per mouse, p=0.68, t(4)=0.4504). (d) Percentage of GABAB1+ cells that do not express TH (n=3 mice, 4 sections per mouse, p=0.30, t(4)=1.191). All data are shown as mean ± s.e.m.
Supplementary Figure 4 Amplitudes of GABAA oIPSCs correlate with electrophysiological properties of GABA neurons and amplitudes of GABAB oIPSCs correlate with electrophysiological properties of dopamine neurons.
(a-c) Example traces demonstrating the AP width (a), firing rate (b), and h-current (c) of a representative GABA (red) and dopamine (blue) neuron. (d) Correlation of GABAA oIPSCs with AP width (n=45 cells, 6 mice). (e) Correlation of GABAA oIPSCs with firing rate (n=45 cells, 6 mice). (f) Correlation of GABAA oIPSCs with h-current (n=45 cells, 6 mice). (g) Correlation of GABAB oIPSCs with AP width (n=64 cells, 12 mice). (h) Correlation of GABAB oIPSCs with firing rate (n=64 cells, 12 mice). (i) Correlation of GABAB oIPSCs with h-current (n=64 cells, 12 mice).
Supplementary Figure 5 Nucleus accumbens inputs preferentially activate GABABRs in dopamine neurons and GABAARs in GABA neurons.
(a) Horizontal brain section containing biocytin-filled cells – two TH+ cells and two TH- cells (scale bar, 60 μm). (b) Representative GABAB oIPSCs from the cells shown in (a). (c) Summary data of GABAB oIPSCs in TH+ and TH- neurons (Vm=-55 mV; n=8 and 10 cells, respectively, 6 mice; unpaired t-test, p<0.01, t(16)=5.502). (d,e) Example (d) and summary (e) of GABAA oIPSCs in VTA dopamine (blue) and GABA (red) neurons using K-gluconate internal solution (Vm=-55 mV; n=15 and 12 cells respectively, 4 mice each, unpaired t-test, p<0.01, t(25)=7.808). (f,g) Example (f) and summary (g) of GABAB oIPSCs in VTA dopamine and GABA neurons (Vm=-55 mV; n=16 and 11 cells respectively, 4 mice each, unpaired t-test, p<0.01, t(25)=7.989). All data are shown as mean ± s.e.m.
Supplementary Figure 6 Evidence for synaptic release of GABA onto GABAB receptors.
(a) Time to onset (10% max current) of GABAB IPSCs while GABA (1M) was iontophoretically released (open circles) at different distances from the soma compared to the time to onset of electrically-evoked GABAB eIPSCs (closed circle, n=9 and 10 cells). (b) Experimental schematic for (c-e). GABAA oIPSCs were evoked in voltage clamp recordings (-70 mV) of VTA GABA neurons. (c) Representative traces of normalized NAc→VTA GABAA oIPSCs in normal artificial cerebrospinal fluid (aCSF) versus dextran-incubated slices. (d) Summary of time to onset for GABAA oIPSCs from normal ACSF versus dextran-incubated slices (n= 8 cells, two-tailed t-test, t(14)=0.18, p=0.86). (e) Summary of time to maximum current for GABAA oIPSCs from normal ACSF versus dextran-incubated slices (n= 8 cells, two-tailed t-test, t(14)=1.85, p=0.09). (f) Experimental schematic for (g-i). GABAB oIPSCs were evoked during voltage clamp recordings (-55 mV) of VTA dopamine neurons. (g) Representative traces of normalized NAc→VTA GABAB oIPSCs onto dopamine neurons in normal aCSF versus dextran-incubated slices. (h) Summary of time to onset for GABAB oIPSCs from normal ACSF versus dextran-incubated slices (n= 9 cells, two-tailed t-test, t(16)=0.32, p=0.75). (i) Summary of time to maximum current for GABAB oIPSCs from normal ACSF versus dextran-incubated slices (n=9 cells, two-tailed t-test, t(16)=0.58, p=0.55). (j) Representative iontophoretic GABAB current before and after dextran application. (k) Time to peak amplitude of iontophoretic GABAB current before and after dextran (n=3 cells, paired t-test, t(2)=7.647, p<0.05). (l) Peak amplitude of iontophoretic currents at different distances from the cell, normalized to the peak amplitude at 0 μm (n=7 cells each, two-way ANOVA, F(4,60)=2.723, p<0.05). (m) Decay time from the peak of iontophoretic currents (n=7 cells each, two-way ANOVA, F(4,55)=9.985, p<0.05) All data are shown as mean ± s.e.m.
Supplementary Figure 7 Nucleus accumbens inputs inhibit dopamine neurons via GABABRs.
(a) Experimental schematic, stimulating NAc→VTA terminals and recording tonic firing in cell-attached mode from VTA dopamine neurons. (b-f) Effect of optical stimulation of NAc→VTA terminals on the normalized firing rate of dopamine cells at various frequencies (1, 2, 5, 10, or 20 Hz). Optical stimulation inhibited dopamine cell firing during baseline (blue, n=10 cells and 4 mice) and during GABAA blockade with picrotoxin (100 μM, grey, n=8 cells and 4 mice), but not during GABAB blockade with CGP 35348 (100 μM, black, n=8 cells and 4 mice). Data are shown as mean ± s.e.m.
Supplementary Figure 8 VTA GABA neurons inhibit dopamine neurons via GABAARs.
(a) Experimental schematic, stimulating VTA GABA neurons and recording tonic firing in cell-attached mode from VTA dopamine neurons. (b-f) Effect of optical stimulation of VTA GABA neurons on the normalized firing rate of dopamine cells at various frequencies (1, 2, 5, 10, or 20 Hz). Optical stimulation inhibited dopamine cell firing during baseline (red, n=10 cells and 4 mice) and during GABAB blockade with CGP 35348 (100 μM, black, n=11 cells and 5 mice), but not during GABAA blockade with picrotoxin (100 μM, grey, n=11 cells and 5 mice). Data are shown as mean ± s.e.m.
Supplementary Figure 9 Deletion of GABABRs from dopamine neurons does not affect general or morphine-induced locomotion.
(a) Summary of locomotor activity in a 30 min open-field test for AAV-Control versus AAV-TH-iCre mice (n=8 mice each, unpaired t-test, t(7)=0.3768, p=0.71). (b) Summary of time spent in the center during the open-field test for AAV Control versus AAV TH-iCre mice (n=8 mice each, unpaired t-test, t(7)=0.88, p=0.40). (c) Locomotor activity in 15 min bins after 10 mg/kg morphine injection. (d) Locomotor activity in 15 min bins after 30 mg/kg morphine injection. All data are shown as mean ± s.e.m.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 (PDF 1402 kb)
Rights and permissions
About this article
Cite this article
Edwards, N., Tejeda, H., Pignatelli, M. et al. Circuit specificity in the inhibitory architecture of the VTA regulates cocaine-induced behavior. Nat Neurosci 20, 438–448 (2017). https://doi.org/10.1038/nn.4482
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4482
This article is cited by
-
NAc-DBS corrects depression-like behaviors in CUMS mouse model via disinhibition of DA neurons in the VTA
Molecular Psychiatry (2024)
-
Activation of RMTg projections to the VTA reverse cocaine-induced molecular adaptation in the reward system
Translational Psychiatry (2024)
-
Paternal cocaine-seeking motivation defines offspring’s vulnerability to addiction by down-regulating GABAergic GABRG3 in the ventral tegmental area
Translational Psychiatry (2024)
-
Ethanol blocks a novel form of iLTD, but not iLTP of inhibitory inputs to VTA GABA neurons
Neuropsychopharmacology (2023)
-
Dichotomous regulation of striatal plasticity by dynorphin
Molecular Psychiatry (2023)