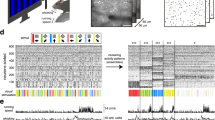
Abstract
Neuronal activity is important for the functional refinement of neuronal circuits in the early visual system. At the level of the cerebral cortex, however, it is still unknown whether the formation of fundamental functions such as orientation selectivity depends on neuronal activity, as it has been difficult to suppress activity throughout development. Using genetic silencing of cortical activity starting before the formation of orientation selectivity, we found that the orientation selectivity of neurons in the mouse visual cortex formed and matured normally despite a strong suppression of both spontaneous and visually evoked activity throughout development. After the orientation selectivity formed, the distribution of the preferred orientations of neurons was reorganized. We found that this process required spontaneous activity, but not visually evoked activity. Thus, the initial formation and maturation of orientation selectivity is largely independent of neuronal activity, and the initial selectivity is subsequently modified depending on neuronal activity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Hubel, D.H. & Wiesel, T.N. Receptive fields of cells in striate cortex of very young, visually inexperienced kittens. J. Neurophysiol. 26, 994–1002 (1963).
Wiesel, T.N. & Hubel, D.H. Ordered arrangement of orientation columns in monkeys lacking visual experience. J. Comp. Neurol. 158, 307–318 (1974).
Miller, K.D., Erwin, E. & Kayser, A. Is the development of orientation selectivity instructed by activity? J. Neurobiol. 41, 44–57 (1999).
Li, Y. et al. Clonally related visual cortical neurons show similar stimulus feature selectivity. Nature 486, 118–121 (2012).
Yu, Y.C. et al. Preferential electrical coupling regulates neocortical lineage-dependent microcircuit assembly. Nature 486, 113–117 (2012).
Ohtsuki, G. et al. Similarity of visual selectivity among clonally related neurons in visual cortex. Neuron 75, 65–72 (2012).
Penn, A.A., Riquelme, P.A., Feller, M.B. & Shatz, C.J. Competition in retinogeniculate patterning driven by spontaneous activity. Science 279, 2108–2112 (1998).
Huberman, A.D., Feller, M.B. & Chapman, B. Mechanisms underlying development of visual maps and receptive fields. Annu. Rev. Neurosci. 31, 479–509 (2008).
Cang, J. et al. Development of precise maps in visual cortex requires patterned spontaneous activity in the retina. Neuron 48, 797–809 (2005).
Huberman, A.D., Speer, C.M. & Chapman, B. Spontaneous retinal activity mediates development of ocular dominance columns and binocular receptive fields in v1. Neuron 52, 247–254 (2006).
Crair, M.C., Gillespie, D.C. & Stryker, M.P. The role of visual experience in the development of columns in cat visual cortex. Science 279, 566–570 (1998).
Sengpiel, F., Stawinski, P. & Bonhoeffer, T. Influence of experience on orientation maps in cat visual cortex. Nat. Neurosci. 2, 727–732 (1999).
Kreile, A.K., Bonhoeffer, T. & Hubener, M. Altered visual experience induces instructive changes of orientation preference in mouse visual cortex. J. Neurosci. 31, 13911–13920 (2011).
Rochefort, N.L. et al. Development of direction selectivity in mouse cortical neurons. Neuron 71, 425–432 (2011).
Kang, E. et al. Visual acuity development and plasticity in the absence of sensory experience. J. Neurosci. 33, 17789–17796 (2013).
Chapman, B. & Stryker, M.P. Development of orientation selectivity in ferret visual cortex and effects of deprivation. J. Neurosci. 13, 5251–5262 (1993).
White, L.E., Coppola, D.M. & Fitzpatrick, D. The contribution of sensory experience to the maturation of orientation selectivity in ferret visual cortex. Nature 411, 1049–1052 (2001).
Yuste, R., Peinado, A. & Katz, L.C. Neuronal domains in developing neocortex. Science 257, 665–669 (1992).
Garaschuk, O., Linn, J., Eilers, J. & Konnerth, A. Large-scale oscillatory calcium waves in the immature cortex. Nat. Neurosci. 3, 452–459 (2000).
Rochefort, N.L. et al. Sparsification of neuronal activity in the visual cortex at eye-opening. Proc. Natl. Acad. Sci. USA 106, 15049–15054 (2009).
Allene, C. & Cossart, R. Early NMDA receptor-driven waves of activity in the developing neocortex: physiological or pathological network oscillations? J. Physiol. (Lond.) 588, 83–91 (2010).
Ackman, J.B., Burbridge, T.J. & Crair, M.C. Retinal waves coordinate patterned activity throughout the developing visual system. Nature 490, 219–225 (2012).
Siegel, F., Heimel, J.A., Peters, J. & Lohmann, C. Peripheral and central inputs shape network dynamics in the developing visual cortex in vivo. Curr. Biol. 22, 253–258 (2012).
Weliky, M. & Katz, L.C. Disruption of orientation tuning in visual cortex by artificially correlated neuronal activity. Nature 386, 680–685 (1997).
Chiu, C. & Weliky, M. Spontaneous activity in developing ferret visual cortex in vivo. J. Neurosci. 21, 8906–8914 (2001).
Ko, H. et al. The emergence of functional microcircuits in visual cortex. Nature 496, 96–100 (2013).
Ko, H., Mrsic-Flogel, T.D. & Hofer, S.B. Emergence of feature-specific connectivity in cortical microcircuits in the absence of visual experience. J. Neurosci. 34, 9812–9816 (2014).
Kuhlman, S.J., Tring, E. & Trachtenberg, J.T. Fast-spiking interneurons have an initial orientation bias that is lost with vision. Nat. Neurosci. 14, 1121–1123 (2011).
Li, Y.T., Ma, W.P., Pan, C.J., Zhang, L.I. & Tao, H.W. Broadening of cortical inhibition mediates developmental sharpening of orientation selectivity. J. Neurosci. 32, 3981–3991 (2012).
Wang, B.S., Feng, L., Liu, M., Liu, X. & Cang, J. Environmental enrichment rescues binocular matching of orientation preference in mice that have a precocious critical period. Neuron 80, 198–209 (2013).
Hoy, J.L. & Niell, C.M. Layer-specific refinement of visual cortex function after eye opening in the awake mouse. J. Neurosci. 35, 3370–3383 (2015).
Dräger, U.C. Receptive fields of single cells and topography in mouse visual cortex. J. Comp. Neurol. 160, 269–290 (1975).
Mank, M. et al. A genetically encoded calcium indicator for chronic in vivo two-photon imaging. Nat. Methods 5, 805–811 (2008).
Girshick, A.R., Landy, M.S. & Simoncelli, E.P. Cardinal rules: visual orientation perception reflects knowledge of environmental statistics. Nat. Neurosci. 14, 926–932 (2011).
Coppola, D.M., Purves, H.R., McCoy, A.N. & Purves, D. The distribution of oriented contours in the real world. Proc. Natl. Acad. Sci. USA 95, 4002–4006 (1998).
Coppola, D.M., White, L.E., Fitzpatrick, D. & Purves, D. Unequal representation of cardinal and oblique contours in ferret visual cortex. Proc. Natl. Acad. Sci. USA 95, 2621–2623 (1998).
Johns, D.C., Marx, R., Mains, R.E., O′Rourke, B. & Marban, E. Inducible genetic suppression of neuronal excitability. J. Neurosci. 19, 1691–1697 (1999).
Mizuno, H., Hirano, T. & Tagawa, Y. Evidence for activity-dependent cortical wiring: formation of interhemispheric connections in neonatal mouse visual cortex requires projection neuron activity. J. Neurosci. 27, 6760–6770 (2007).
Ohki, K., Chung, S., Ch′ng, Y.H., Kara, P. & Reid, R.C. Functional imaging with cellular resolution reveals precise micro-architecture in visual cortex. Nature 433, 597–603 (2005).
Niculescu, D. & Lohmann, C. Gap junctions in developing thalamic and neocortical neuronal networks. Cereb. Cortex 24, 3097–3106 (2013).
Yamada, A. et al. Role of pre- and postsynaptic activity in thalamocortical axon branching. Proc. Natl. Acad. Sci. USA 107, 7562–7567 (2010).
Sohya, K., Kameyama, K., Yanagawa, Y., Obata, K. & Tsumoto, T. GABAergic neurons are less selective to stimulus orientation than excitatory neurons in layer II/III of visual cortex, as revealed by in vivo functional Ca2+ imaging in transgenic mice. J. Neurosci. 27, 2145–2149 (2007).
Kerlin, A.M., Andermann, M.L., Berezovskii, V.K. & Reid, R.C. Broadly tuned response properties of diverse inhibitory neuron subtypes in mouse visual cortex. Neuron 67, 858–871 (2010).
Hofer, S.B. et al. Differential connectivity and response dynamics of excitatory and inhibitory neurons in visual cortex. Nat. Neurosci. 14, 1045–1052 (2011).
Hooks, B.M. & Chen, C. Distinct roles for spontaneous and visual activity in remodeling of the retinogeniculate synapse. Neuron 52, 281–291 (2006).
Marshel, J.H., Kaye, A.P., Nauhaus, I. & Callaway, E.M. Anterior-posterior direction opponency in the superficial mouse lateral geniculate nucleus. Neuron 76, 713–720 (2012).
Li, Y.T., Ibrahim, L.A., Liu, B.H., Zhang, L.I. & Tao, H.W. Linear transformation of thalamocortical input by intracortical excitation. Nat. Neurosci. 16, 1324–1330 (2013).
Cruz-Martín, A. et al. A dedicated circuit links direction-selective retinal ganglion cells to the primary visual cortex. Nature 507, 358–361 (2014).
Elstrott, J. et al. Direction selectivity in the retina is established independent of visual experience and cholinergic retinal waves. Neuron 58, 499–506 (2008).
Smith, G.B. & Fitzpatrick, D. Specifying cortical circuits: a role for cell lineage. Neuron 75, 4–5 (2012).
Shcherbo, D. et al. Bright far-red fluorescent protein for whole-body imaging. Nat. Methods 4, 741–746 (2007).
Drobizhev, M., Makarov, N.S., Tillo, S.E., Hughes, T.E. & Rebane, A. Two-photon absorption properties of fluorescent proteins. Nat. Methods 8, 393–399 (2011).
Mizuno, H., Hirano, T. & Tagawa, Y. Pre-synaptic and post-synaptic neuronal activity supports the axon development of callosal projection neurons during different post-natal periods in the mouse cerebral cortex. Eur. J. Neurosci. 31, 410–424 (2010).
Hagihara, K.M. & Ohki, K. Long-term down-regulation of GABA decreases orientation selectivity without affecting direction selectivity in mouse primary visual cortex. Front. Neural Circuits 7, 28 (2013).
Peirce, J.W. PsychoPy–Psychophysics software in Python. J. Neurosci. Methods 162, 8–13 (2007).
Helmchen, F., Imoto, K. & Sakmann, B. Ca2+ buffering and action potential-evoked Ca2+ signaling in dendrites of pyramidal neurons. Biophys. J. 70, 1069–1081 (1996).
Kitamura, K., Judkewitz, B., Kano, M., Denk, W. & Hausser, M. Targeted patch-clamp recordings and single-cell electroporation of unlabeled neurons in vivo. Nat. Methods 5, 61–67 (2008).
Komai, S., Denk, W., Osten, P., Brecht, M. & Margrie, T.W. Two-photon targeted patching (TPTP) in vivo. Nat. Protoc. 1, 647–652 (2006).
Glass, G.V., Peckham, P.D. & Sanders, J.R. Consequences of failure to meet the assumptions underlying the fixed effects analysis of variance and covariance. Rev. Educ. Res. 42, 237–288 (1972).
Acknowledgements
We thank T. Yokomizo (Juntendo University) for help and advice on molecular biology works, such as plasmids construction, Y. Tezuka (Kyoto University) for sharing the information about Tet-gene expression system development and histology works, M.H. Histed (Harvard Medical School) for reading the manuscript, K. Kitamura (University of Yamanashi), M. Kano and A. Takeuchi (University of Tokyo) for showing cell-attached recordings, S. Kondo (Kyushu University) for electrophysiology set up, K. Hayashi (Kyushu University) for plasmids construction, A. Honda and Y. Sono (Kyushu University) for animal care and genotyping, T. Hirano (Kyoto University), all of the members of Ohki laboratory for support and discussion, and the Research Support Center, Graduate School of Medical Sciences, Kyushu University for technical support. This work was supported by grants from CREST-JST (to K.O. and Y.T.), JSPS KAKENHI (grant number 25221001 to K.O., 23500388 to Y.T.), JST, Strategic International Research Cooperative Program, SCIP (to K.O.), Grant-in-Aid for Scientific Research on Innovative Areas, “Glial assembly: a new regulatory machinery of brain function and disorders” (25117004 to K.O.) and “Neural Diversity and Neocortical Organization” (23123508 and 25123707 to Y.T.), grants from the Ichiro Kanehara Foundation for the Promotion of Medical Sciences and Medical Care, and the Uehara Memorial Foundation (to T.Y.). A part of this work was carried out under the Brain/MINDS by the MEXT of Japan. K.M.H. was supported by Takeda Science Foundation. T. M. was supported by JSPS Research Fellowship for Young Scientists(201503597).
Author information
Authors and Affiliations
Contributions
K.M.H., Y.T. and K.O. designed the research. K.M.H. performed most of the experiments and analyzed the data. T.M. performed two-photon imaging experiments, cell-attached recording experiments and wide-field imaging experiments, and analyzed the wide-field imaging data. Y.T. designed and constructed plasmids, performed in utero electroporation, and helped K.M.H. for histology experiments. T.Y. performed two-photon imaging experiments and in utero electroporation. K.M.H., Y.T., T.Y. and K.O. wrote the manuscript. Y.T. and K.O. supervised the entire project. All of the authors discussed and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–16 and Supplementary Table 1 (PDF 12777 kb)
Rights and permissions
About this article
Cite this article
Hagihara, K., Murakami, T., Yoshida, T. et al. Neuronal activity is not required for the initial formation and maturation of visual selectivity. Nat Neurosci 18, 1780–1788 (2015). https://doi.org/10.1038/nn.4155
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4155
This article is cited by
-
Intercalated amygdala clusters orchestrate a switch in fear state
Nature (2021)
-
Natural images are reliably represented by sparse and variable populations of neurons in visual cortex
Nature Communications (2020)
-
Characterisation of nonlinear receptive fields of visual neurons by convolutional neural network
Scientific Reports (2019)
-
Mice use robust and common strategies to discriminate natural scenes
Scientific Reports (2018)
-
Causal evidence for retina-dependent and -independent visual motion computations in mouse cortex
Nature Neuroscience (2017)