Abstract
The microtubule-associated protein tau has been implicated in the pathogenesis of Alzheimer's disease (AD) and other neurodegenerative disorders. Reducing tau levels ameliorates AD-related synaptic, network, and behavioral abnormalities in transgenic mice expressing human amyloid precursor protein (hAPP). We used mass spectrometry to characterize the post-translational modification of endogenous tau isolated from wild-type and hAPP mice. We identified seven types of tau modifications at 63 sites in wild-type mice. Wild-type and hAPP mice had similar modifications, supporting the hypothesis that neuronal dysfunction in hAPP mice is enabled by physiological forms of tau. Our findings provide clear evidence for acetylation and ubiquitination of the same lysine residues; some sites were also targeted by lysine methylation. Our findings refute the hypothesis of extensive O-linked N-acetylglucosamine (O-GlcNAc) modification of endogenous tau. The complex post-translational modification of physiological tau suggests that tau is regulated by diverse mechanisms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Morris, M., Maeda, S., Vossel, K. & Mucke, L. The many faces of tau. Neuron 70, 410–426 (2011).
Huang, Y. & Mucke, L. Alzheimer mechanisms and therapeutic strategies. Cell 148, 1204–1222 (2012).
Roberson, E.D. et al. Reducing endogenous tau ameliorates amyloid beta–induced deficits in an Alzheimer’s disease mouse model. Science 316, 750–754 (2007).
Roberson, E.D. et al. Amyloid-β/Fyn-induced synaptic, network and cognitive impairments depend on tau levels in multiple mouse models of Alzheimer’s disease. J. Neurosci. 31, 700–711 (2011).
Ittner, L.M. et al. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer’s disease mouse models. Cell 142, 387–397 (2010).
Seward, M.E. et al. Amyloid-β signals through tau to drive ectopic neuronal cell cycle re-entry in Alzheimer’s disease. J. Cell Sci. 126, 1278–1286 (2013).
Suberbielle, E. et al. Physiologic brain activity causes DNA double-strand breaks in neurons, with exacerbation by amyloid-β. Nat. Neurosci. 16, 613–621 (2013).
DeVos, S.L. et al. Antisense reduction of tau in adult mice protects against seizures. J. Neurosci. 33, 12887–12897 (2013).
Gheyara, A.L. et al. Tau reduction prevents disease in a mouse model of Dravet syndrome. Ann. Neurol. 76, 443–456 (2014).
Holth, J.K. et al. Tau loss attenuates neuronal network hyperexcitability in mouse and Drosophila genetic models of epilepsy. J. Neurosci. 33, 1651–1659 (2013).
Li, Z., Hall, A.M., Kelinske, M. & Roberson, E.D. Seizure resistance without parkinsonism in aged mice after tau reduction. Neurobiol. Aging 35, 2617–2624 (2014).
Zempel, H. et al. Amyloid-β oligomers induce synaptic damage via Tau-dependent microtubule severing by TTLL6 and spastin. EMBO J. 32, 2920–2937 (2013).
Zempel, H., Thies, E., Mandelkow, E. & Mandelkow, E.-M. Abeta oligomers cause localized Ca2+ elevation, missorting of endogenous Tau into dendrites, Tau phosphorylation and destruction of microtubules and spines. J. Neurosci. 30, 11938–11950 (2010).
Funk, K.E. et al. Lysine methylation is an endogenous post-translational modification of tau protein in human brain and a modulator of aggregation propensity. Biochem. J. 462, 77–88 (2014).
Guo, A. et al. Immunoaffinity enrichment and mass spectrometry analysis of protein methylation. Mol. Cell. Proteomics 13, 372–387 (2014).
Thomas, S.N. et al. Dual modification of Alzheimer’s disease PHF-tau protein by lysine methylation and ubiquitylation: a mass spectrometry approach. Acta Neuropathol. 123, 105–117 (2012).
Liu, F., Iqbal, K., Grundke-Iqbal, I., Hart, G.W. & Gong, C.-X. O-GlcNAcylation regulates phosphorylation of tau: a mechanism involved in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 101, 10804–10809 (2004).
Yuzwa, S.A. et al. A potent mechanism-inspired O-GlcNAcase inhibitor that blocks phosphorylation of tau in vivo. Nat. Chem. Biol. 4, 483–490 (2008).
Arnold, C.S. et al. The microtubule-associated protein tau is extensively modified with O-linked N-acetylglucosamine. J. Biol. Chem. 271, 28741–28744 (1996).
Wang, Z. et al. Enrichment and site mapping of O-linked N-acetylglucosamine by a combination of chemical/enzymatic tagging, photochemical cleavage and electron transfer dissociation mass spectrometry. Mol. Cell. Proteomics 9, 153–160 (2010).
Hanger, D.P. et al. Novel phosphorylation sites in tau from Alzheimer brain support a role for casein kinase 1 in disease pathogenesis. J. Biol. Chem. 282, 23645–23654 (2007).
Cripps, D. et al. Alzheimer disease-specific conformation of hyperphosphorylated paired helical filament-Tau is polyubiquitinated through Lys-48, Lys-11 and Lys-6 ubiquitin conjugation. J. Biol. Chem. 281, 10825–10838 (2006).
Morishima-Kawashima, M. et al. Ubiquitin is conjugated with amino-terminally processed tau in paired helical filaments. Neuron 10, 1151–1160 (1993).
Cohen, T.J. et al. The acetylation of tau inhibits its function and promotes pathological tau aggregation. Nat. Commun. 2, 252 (2011).
Cook, C. et al. Acetylation of the KXGS motifs in tau is a critical determinant in modulation of tau aggregation and clearance. Hum. Mol. Genet. 23, 104–116 (2014).
Min, S.-W. et al. Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron 67, 953–966 (2010).
Baker, P.R., Trinidad, J.C. & Chalkley, R.J. Modification site localization scoring integrated into a search engine. Mol. Cell Proteomics 10, M111.008078 (2011).
Beausoleil, S.A., Villén, J., Gerber, S.A., Rush, J. & Gygi, S.P. A probability-based approach for high-throughput protein phosphorylation analysis and site localization. Nat. Biotechnol. 24, 1285–1292 (2006).
Hornbeck, P.V. et al. PhosphoSitePlus: a comprehensive resource for investigating the structure and function of experimentally determined post-translational modifications in man and mouse. Nucleic Acids Res. 40, D261–D270 (2012).
Bradshaw, R.A., Medzihradszky, K.F. & Chalkley, R.J. Protein PTMs: post-translational modifications or pesky trouble makers? J. Mass Spectrom. 45, 1095–1097 (2010).
Biggar, K.K. & Li, S.S.-C. Non-histone protein methylation as a regulator of cellular signaling and function. Nat. Rev. Mol. Cell Biol. 16, 5–17 (2015).
Yuzwa, S.A. et al. Increasing O-GlcNAc slows neurodegeneration and stabilizes tau against aggregation. Nat. Chem. Biol. 8, 393–399 (2012).
Borghgraef, P. et al. Increasing brain protein O-GlcNAc-ylation mitigates breathing defects and mortality of Tau.P301L mice. PLoS ONE 8, e84442 (2013).
Liu, F. et al. Reduced O-GlcNAcylation links lower brain glucose metabolism and tau pathology in Alzheimer’s disease. Brain 132, 1820–1832 (2009).
Yuzwa, S.A. et al. Mapping O-GlcNAc modification sites on tau and generation of a site-specific O-GlcNAc tau antibody. Amino Acids 40, 857–868 (2011).
Hoover, B.R. et al. Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron 68, 1067–1081 (2010).
Goedert, M. & Jakes, R. Expression of separate isoforms of human tau protein: correlation with the tau pattern in brain and effects on tubulin polymerization. EMBO J. 9, 4225–4230 (1990).
McMillan, P. et al. Tau isoform regulation is region- and cell-specific in mouse brain. J. Comp. Neurol. 511, 788–803 (2008).
Yang, X.-J. & Seto, E. Lysine acetylation: codified crosstalk with other post-translational modifications. Mol. Cell 31, 449–461 (2008).
Biernat, J. & Mandelkow, E.M. The development of cell processes induced by tau protein requires phosphorylation of serine 262 and 356 in the repeat domain and is inhibited by phosphorylation in the proline-rich domains. Mol. Biol. Cell 10, 727–740 (1999).
Biernat, J., Gustke, N., Drewes, G., Mandelkow, E.M. & Mandelkow, E. Phosphorylation of Ser262 strongly reduces binding of tau to microtubules: distinction between PHF-like immunoreactivity and microtubule binding. Neuron 11, 153–163 (1993).
von Bergen, M. et al. Assembly of tau protein into Alzheimer paired helical filaments depends on a local sequence motif ((306)VQIVYK(311)) forming beta structure. Proc. Natl. Acad. Sci. USA 97, 5129–5134 (2000).
von Bergen, M. et al. Mutations of tau protein in frontotemporal dementia promote aggregation of paired helical filaments by enhancing local beta-structure. J. Biol. Chem. 276, 48165–48174 (2001).
Brandt, R., Léger, J. & Lee, G. Interaction of tau with the neural plasma membrane mediated by tau’s amino-terminal projection domain. J. Cell Biol. 131, 1327–1340 (1995).
Reynolds, C.H. et al. Phosphorylation regulates tau interactions with Src homology 3 domains of phosphatidylinositol 3-kinase, phospholipase Cgamma1, Grb2 and Src family kinases. J. Biol. Chem. 283, 18177–18186 (2008).
Sultan, A. et al. Nuclear tau, a key player in neuronal DNA protection. J. Biol. Chem. 286, 4566–4575 (2011).
Mandelkow, E.M. et al. Tau domains, phosphorylation, and interactions with microtubules. Neurobiol. Aging 16, 355–362 (1995).
Sturchler-Pierrat, C. et al. Two amyloid precursor protein transgenic mouse models with Alzheimer disease–like pathology. Proc. Natl. Acad. Sci. USA 94, 13287–13292 (1997).
Simón, A.-M. et al. Overexpression of wild-type human APP in mice causes cognitive deficits and pathological features unrelated to Abeta levels. Neurobiol. Dis. 33, 369–378 (2009).
Köpke, E. et al. Microtubule-associated protein tau. Abnormal phosphorylation of a non-paired helical filament pool in Alzheimer disease. J. Biol. Chem. 268, 24374–24384 (1993).
Mucke, L. et al. High-level neuronal expression of Aβ1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J. Neurosci. 20, 4050–4058 (2000).
Dawson, H.N. et al. Inhibition of neuronal maturation in primary hippocampal neurons from tau deficient mice. J. Cell Sci. 114, 1179–1187 (2001).
Planel, E. et al. Anesthesia leads to tau hyperphosphorylation through inhibition of phosphatase activity by hypothermia. J. Neurosci. 27, 3090–3097 (2007).
Ivanovova, N., Handzusova, M., Hanes, J., Kontsekova, E. & Novak, M. High-yield purification of fetal tau preserving its structure and phosphorylation pattern. J. Immunol. Methods 339, 17–22 (2008).
Morris, M. et al. Age-appropriate cognition and subtle dopamine-independent motor deficits in aged tau knockout mice. Neurobiol. Aging 34, 1523–1529 (2013).
Trinidad, J.C., Thalhammer, A., Specht, C.G., Schoepfer, R. & Burlingame, A.L. Phosphorylation state of postsynaptic density proteins. J. Neurochem. 92, 1306–1316 (2005).
Trinidad, J.C., Specht, C.G., Thalhammer, A., Schoepfer, R. & Burlingame, A.L. Comprehensive identification of phosphorylation sites in postsynaptic density preparations. Mol. Cell. Proteomics 5, 914–922 (2006).
Vossel, K.A. et al. Tau reduction prevents Aβ-induced axonal transport deficits by blocking activation of GSK3β. J. Cell Biol. 209, 419–433 (2015).
Chalkley, R.J., Thalhammer, A., Schoepfer, R. & Burlingame, A.L. Identification of protein O-GlcNAcylation sites using electron transfer dissociation mass spectrometry on native peptides. Proc. Natl. Acad. Sci. USA 106, 8894–8899 (2009).
Trinidad, J.C. et al. Global identification and characterization of both O-GlcNAcylation and phosphorylation at the murine synapse. Mol. Cell. Proteomics 11, 215–229 (2012).
Vosseller, K. et al. O-linked N-acetylglucosamine proteomics of postsynaptic density preparations using lectin weak affinity chromatography and mass spectrometry. Mol. Cell. Proteomics 5, 923–934 (2006).
Olsen, J.V. et al. Parts per million mass accuracy on an Orbitrap mass spectrometer via lock mass injection into a C-trap. Mol. Cell. Proteomics 4, 2010–2021 (2005).
Chalkley, R.J., Baker, P.R., Medzihradszky, K.F., Lynn, A.J. & Burlingame, A.L. In-depth analysis of tandem mass spectrometry data from disparate instrument types. Mol. Cell. Proteomics 7, 2386–2398 (2008).
Elias, J.E. & Gygi, S.P. Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat. Methods 4, 207–214 (2007).
Guan, S., Price, J.C., Prusiner, S.B., Ghaemmaghami, S. & Burlingame, A.L. A data processing pipeline for mammalian proteome dynamics studies using stable isotope metabolic labeling. Mol. Cell Proteomics 10, M111.010728 (2011).
Rudrabhatla, P., Jaffe, H. & Pant, H.C. Direct evidence of phosphorylated neuronal intermediate filament proteins in neurofibrillary tangles (NFTs): phosphoproteomics of Alzheimer’s NFTs. FASEB J. 25, 3896–3905 (2011).
Tavares, I.A. et al. Prostate-derived sterile 20-like kinases (PSKs/TAOKs) phosphorylate tau protein and are activated in tangle-bearing neurons in Alzheimer disease. J. Biol. Chem. 288, 15418–15429 (2013).
Acknowledgements
We thank M. Finucane for help with the statistical analysis, S. Lee, W. Guo, J. Kang, X. Wang, D. Kim and G.-Q. Yu for technical assistance, S. Ordway for editorial assistance, and M. Dela Cruz and A. Cheung for administrative assistance. The study was supported by US National Institutes of Health grants to L.M. (NS041787) and to A.L.B. (GM103481).
Author information
Authors and Affiliations
Contributions
M.M. designed and conducted the mouse experiments, the statistical analyses and wrote the manuscript. G.M.K. analyzed and curated the PTM mapping data, conducted the quantitative mass spectrometry experiments and wrote the manuscript. S.M. conducted PSD fractionation and western blots, and wrote the manuscript. J.C.T. assisted in mass spectrometry method development and wrote the manuscript. A.I. conducted PTM mapping experiments by mass spectrometry. A.L.B. and L.M. supervised the project and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
L.M. receives research support from Bristol-Myers Squibb.
Integrated supplementary information
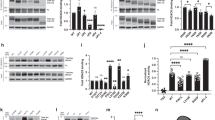
Supplementary Figure 1 Comparison of post-translational modifications in wildtype and hAPP mice.
Tau was isolated from hAPP and wildtype mice and the levels of all quantifiable post-translational modifications were assessed by mass spectrometry. Wildtype tau modification levels in each cohort were defined as 1.0. (a) Tau was isolated from whole cortical and hippocampal lysates either by selective solubility in perchloric acid from wildtype or hyperactive hAPP mice (Cohort A, n = 3 mice per group, 6 months of age) or by immunoprecipitation in randomly selected wildtype and hAPP mice (Cohort B, n=12 mice per group, 7–10 months of age). (b) Tau was immunoprecipitated from PSD fractions of three cohorts of randomly selected mice (Cohorts B, D, and E, n = 10 or 12 mice per group, ages 7–10 months, Supplementary Table 4). After correcting Student’s t-test p-values for multiple comparisons using a Benjamini-Hochberg correction, no p-values were ≤0.05. p=0.06 for pS416 in Cohort A (a) and p=0.29 for tri-p198-210 in Cohort E (b) prior to correction for multiple comparisons. A Student's t-test with Welch's correction was used if the variance differed significantly between groups. Modified amino acids are shown that were unambiguously assigned and conserved in human tau 441. Multiple unambiguous modifications are listed with ‘+’ between residues. If the site was ambiguous, a peptide sequence containing the modified sites was indicated with ‘di-‘ or ‘tri-‘ indicating doubly and triply modified peptides, respectively. Ac, acetylation; GG, GlyGly; Me, methylation; p, phosphorylation; WT, wildtype; hAPP, human amyloid precursor protein transgenic; PCA, isolated by perchloric acid solubility; IP, immunoprecipitated. Quantitative values are means ± SEM.
Supplementary Figure 2 Comparison of S400 O-GlcNAc modification of tau in wildtype and hAPP mice.
(a) O-GlcNAc modification of tau at S400 (gS400) was measured relative to total tau by western blotting using Ab3925 and Tau5 antibodies in cortical and hippocampal homogenates. (Cohort C, n = 4 mice per group, 7–8 months of age, Supplementary Table 4). Mice lacking endogenous tau served as negative controls. (b) Quantification of S400 O-GlcNAc modification relative to total tau (t(6) = 1.251, p = 0.257 by unpaired, two-tailed Student’s t-test). Quantitative values are means ± SEM.
Supplementary Figure 3 Comparison of PHF1 immunoreactivity in the PSD fraction of wildtype and hAPP mice.
PSD fractions were prepared from combined cortex and hippocampus homogenates. (a) Phosphorylation of tau at the PHF1 epitope (pS396, pS404) in Cohort F (n = 5 WT and 6 hAPP mice per group, 5 months of age, Supplementary Table 4). Similar results were obtained in independent groups of mice from Cohort G (n = 6 mice per group, 5-7 months of age, data not shown). Mice lacking endogenous tau served as negative controls, and actin was used as a loading control. The panels were cropped from the same image of a single membrane to facilitate the comparison of different genotypes. (b) Quantification of PHF1 relative to actin (t(9) = 0.053, p = 0.958 by unpaired two-tailed Student’s t-test). Quantitative values are means ± SEM.
Supplementary Figure 4 Full-length images of the blots presented in the main text.
Western blot corresponding to Figure 2a showing PSD95, tau and α-synuclein levels in two different mice from a replicate cohort at each step of post-synaptic density fractionation.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4 (PDF 618 kb)
Supplementary Table 1
Summary of experiments used to identify post-translational modifications of tau (reporting data available for viewing through MASSive). (XLSX 17 kb)
Supplementary Table 2
Peptide identifications for modified and unmodified species of tau (data also provided in MS-Viewer format). (XLSX 69 kb)
Supplementary Table 3
Summary of OGlcNAc-Modified Peptides Identified by LWAC Enrichment. (XLSX 113 kb)
Supplementary Table 4
Mouse cohorts used for quantitative experiments. (XLSX 10 kb)
Supplementary Table 5
Quantified tau peptides from hippocampal and cortical whole lysate of cohort A (data also provided in MS-Viewer format). (XLSX 13 kb)
Supplementary Table 6
Quantified tau peptides from hippocampal and cortical whole lysate, and the post-synaptic density fraction of cohort B (data also provided in MS-Viewer format). (XLSX 20 kb)
Supplementary Table 7
Quantified tau peptides from post-synaptic density fraction of cohort D (data also provided in MS-Viewer format). (XLSX 16 kb)
Supplementary Table 8
Quantified tau peptides from post-synaptic density fraction of cohort E (data also provided in MS-Viewer format). (XLSX 22 kb)
Supplementary Table 9
Comparison of tau modifications identified in this study and previously reported modifications identified in mouse/rat or human brain tissue. (XLSX 28 kb)
Rights and permissions
About this article
Cite this article
Morris, M., Knudsen, G., Maeda, S. et al. Tau post-translational modifications in wild-type and human amyloid precursor protein transgenic mice. Nat Neurosci 18, 1183–1189 (2015). https://doi.org/10.1038/nn.4067
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4067
This article is cited by
-
Traumatic Brain Injury as a Risk Factor for Alzheimer’s Disease and Potential for Pathogenetic Therapy
Neuroscience and Behavioral Physiology (2024)
-
Cis P-tau is a central circulating and placental etiologic driver and therapeutic target of preeclampsia
Nature Communications (2023)
-
Tau forms synaptic nano-biomolecular condensates controlling the dynamic clustering of recycling synaptic vesicles
Nature Communications (2023)
-
Detection, visualization and quantification of protein complexes in human Alzheimer’s disease brains using proximity ligation assay
Scientific Reports (2023)
-
The Involvement of Post-Translational Modifications in Regulating the Development and Progression of Alzheimer’s Disease
Molecular Neurobiology (2023)