Abstract
Neuregulin 1 type III is processed following regulated intramembrane proteolysis, which allows communication from the plasma membrane to the nucleus. We found that the intracellular domain of neuregulin 1 type III upregulated the prostaglandin D2 synthase (L-pgds, also known as Ptgds) gene, which, together with the G protein–coupled receptor Gpr44, forms a previously unknown pathway in PNS myelination. Neuronal L-PGDS is secreted and produces the PGD2 prostanoid, a ligand of Gpr44. We found that mice lacking L-PGDS were hypomyelinated. Consistent with this, specific inhibition of L-PGDS activity impaired in vitro myelination and caused myelin damage. Furthermore, in vivo ablation and in vitro knockdown of glial Gpr44 impaired myelination. Finally, we identified Nfatc4, a key transcription factor for myelination, as one of the downstream effectors of PGD2 activity in Schwann cells. Thus, L-PGDS and Gpr44 are previously unknown components of an axo-glial interaction that controls PNS myelination and possibly myelin maintenance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Accession codes
Change history
17 November 2014
In the version of this article initially published online, a P value was incorrect on p. 5, first full paragraph. It read “We found similar alterations in 6-month-old sciatic nerves of L-pgds−/− mice, although the difference was not significant (P = 0.649; Supplementary Table 2).” The correct P value is 0.0649. Also, the first Results subheading read “NRG1 type III is cleaved and activates L-PGDS by γ-secretase”; it should have read “NRG1 type III is cleaved by γ-secretase and activates L-PGDS.” The errors have been corrected for the print, PDF and HTML versions of this article.
References
Birchmeier, C. & Nave, K.A. Neuregulin-1, a key axonal signal that drives Schwann cell growth and differentiation. Glia 56, 1491–1497 (2008).
Willem, M. et al. Control of peripheral nerve myelination by the beta-secretase BACE1. Science 314, 664–666 (2006).
Hu, X. et al. Bace1 modulates myelination in the central and peripheral nervous system. Nat. Neurosci. 9, 1520–1525 (2006).
La Marca, R. et al. TACE (ADAM17) inhibits Schwann cell myelination. Nat. Neurosci. 14, 857–865 (2011).
Bao, J., Wolpowitz, D., Role, L.W. & Talmage, D.A. Back signaling by the Nrg-1 intracellular domain. J. Cell Biol. 161, 1133–1141 (2003).
Bao, J. et al. Activity-dependent transcription regulation of PSD-95 by neuregulin-1 and Eos. Nat. Neurosci. 7, 1250–1258 (2004).
Chen, Y., Hancock, M.L., Role, L.W. & Talmage, D.A. Intramembranous valine linked to schizophrenia is required for neuregulin 1 regulation of the morphological development of cortical neurons. J. Neurosci. 30, 9199–9208 (2010).
Urade, Y. & Eguchi, N. Lipocalin-type and hematopoietic prostaglandin D synthases as a novel example of functional convergence. Prostaglandins Other Lipid Mediat. 68–69, 375–382 (2002).
Giacomelli, S., Leone, M.G., Grima, J., Silvestrini, B. & Cheng, C.Y. Astrocytes synthesize and secrete prostaglandin D synthetase in vitro. Biochim. Biophys. Acta 1310, 269–276 (1996).
Samy, E.T. et al. Sertoli cell prostaglandin D2 synthetase is a multifunctional molecule: its expression and regulation. Endocrinology 141, 710–721 (2000).
Nagata, N. et al. De novo synthesis, uptake and proteolytic processing of lipocalin-type prostaglandin D synthase, beta-trace, in the kidneys. FEBS J. 276, 7146–7158 (2009).
Tootle, T.L. Genetic insights into the in vivo functions of prostaglandin signaling. Int. J. Biochem. Cell Biol. 45, 1629–1632 (2013).
Haskew-Layton, R.E., Payappilly, J.B., Xu, H., Bennett, S.A. & Ratan, R.R. 15-Deoxy-Delta12,14-prostaglandin J2 (15d-PGJ2) protects neurons from oxidative death via an Nrf2 astrocyte-specific mechanism independent of PPARgamma. J. Neurochem. 124, 536–547 (2013).
Kerr, B.J., Girolami, E.I., Ghasemlou, N., Jeong, S.Y. & David, S. The protective effects of 15-deoxy-delta-(12,14)-prostaglandin J2 in spinal cord injury. Glia 56, 436–448 (2008).
Urade, Y. & Hayaishi, O. Prostaglandin D synthase: structure and function. Vitam. Horm. 58, 89–120 (2000).
Kostenis, E. & Ulven, T. Emerging roles of DP and CRTH2 in allergic inflammation. Trends Mol. Med. 12, 148–158 (2006).
Beuckmann, C.T. et al. Cellular localization of lipocalin-type prostaglandin D synthase (beta-trace) in the central nervous system of the adult rat. J. Comp. Neurol. 428, 62–78 (2000).
Kao, S.C. et al. Calcineurin/NFAT signaling is required for neuregulin-regulated Schwann cell differentiation. Science 323, 651–654 (2009).
Grimwood, S. et al. Determination of guinea-pig cortical gamma-secretase activity ex vivo following the systemic administration of a gamma-secretase inhibitor. Neuropharmacology 48, 1002–1011 (2005).
Beuckmann, C.T. et al. Lipocalin-type prostaglandin D synthase (beta-trace) is located in pigment epithelial cells of rat retina and accumulates within interphotoreceptor matrix. J. Neurosci. 16, 6119–6124 (1996).
Mohri, I. et al. Prostaglandin D2–mediated microglia/astrocyte interaction enhances astrogliosis and demyelination in twitcher. J. Neurosci. 26, 4383–4393 (2006).
Subra, C. et al. Exosomes account for vesicle-mediated transcellular transport of activatable phospholipases and prostaglandins. J. Lipid Res. 51, 2105–2120 (2010).
Irikura, D. et al. Biochemical, functional, and pharmacological characterization of AT-56, an orally active and selective inhibitor of lipocalin-type prostaglandin D synthase. J. Biol. Chem. 284, 7623–7630 (2009).
Vesin, M.F., Urade, Y., Hayaishi, O. & Droz, B. Neuronal and glial prostaglandin D synthase isozymes in chick dorsal root ganglia: a light and electron microscopic immunocytochemical study. J. Neurosci. 15, 470–476 (1995).
Ragolia, L. et al. Accelerated glucose intolerance, nephropathy, and atherosclerosis in prostaglandin D2 synthase knock-out mice. J. Biol. Chem. 280, 29946–29955 (2005).
Qu, W.M. et al. Lipocalin-type prostaglandin D synthase produces prostaglandin D2 involved in regulation of physiological sleep. Proc. Natl. Acad. Sci. USA 103, 17949–17954 (2006).
Kanekiyo, T. et al. Lipocalin-type prostaglandin D synthase/beta-trace is a major amyloid beta-chaperone in human cerebrospinal fluid. Proc. Natl. Acad. Sci. USA 104, 6412–6417 (2007).
Moniot, B. et al. The PGD2 pathway, independently of FGF9, amplifies SOX9 activity in Sertoli cells during male sexual differentiation. Development 136, 1813–1821 (2009).
Raff, M.C., Abney, E., Brockes, J.P. & Hornby-Smith, A. Schwann cell growth factors. Cell 15, 813–822 (1978).
Monje, P.V., Athauda, G. & Wood, P.M. Protein kinase A–mediated gating of neuregulin-dependent ErbB2-ErbB3 activation underlies the synergistic action of cAMP on Schwann cell proliferation. J. Biol. Chem. 283, 34087–34100 (2008).
Taveggia, C. et al. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron 47, 681–694 (2005).
Ogata, T. et al. Opposing extracellular signal–regulated kinase and Akt pathways control Schwann cell myelination. J. Neurosci. 24, 6724–6732 (2004).
Newbern, J.M. et al. Specific functions for ERK/MAPK signaling during PNS development. Neuron 69, 91–105 (2011).
Lal, M. & Caplan, M. Regulated intramembrane proteolysis: signaling pathways and biological functions. Physiology (Bethesda) 26, 34–44 (2011).
Harizi, H. The immunobiology of prostanoid receptor signaling in connecting innate and adaptive immunity. Biomed. Res. Int. 2013, 683405 (2013).
Taketomi, Y. et al. Mast cell maturation is driven via a group III phospholipase A2-prostaglandin D2–DP1 receptor paracrine axis. Nat. Immunol. 14, 554–563 (2013).
Tin, A. et al. Genome-wide significant locus of beta-trace protein, a novel kidney function biomarker, identified in European and African Americans. Nephrol. Dial. Transplant. 28, 1497–1504 (2013).
Greenberg, M.E., Xu, B., Lu, B. & Hempstead, B.L. New insights in the biology of BDNF synthesis and release: implications in CNS function. J. Neurosci. 29, 12764–12767 (2009).
Evans, S.F. et al. Neuronal brain-derived neurotrophic factor is synthesized in excess, with levels regulated by sortilin-mediated trafficking and lysosomal degradation. J. Biol. Chem. 286, 29556–29567 (2011).
Nicholson, J.D. et al. PGJ(2) provides prolonged CNS stroke protection by reducing white matter edema. PLoS ONE 7, e50021 (2012).
Kipanyula, M.J. et al. Calcineurin-nuclear factor of activated T cells regulation of Krox-20 expression in Schwann cells requires elevation of intracellular cyclic AMP. J. Neurosci. Res. 91, 105–115 (2013).
Hirahara, Y. et al. G protein-coupled receptor 30 contributes to improved remyelination after cuprizone-induced demyelination. Glia 61, 420–431 (2013).
Hennen, S. et al. Decoding signaling and function of the orphan G protein–coupled receptor GPR17 with a small-molecule agonist. Sci. Signal. 6, ra93 (2013).
Monk, K.R. et al. A G protein–coupled receptor is essential for Schwann cells to initiate myelination. Science 325, 1402–1405 (2009).
Monk, K.R., Oshima, K., Jors, S., Heller, S. & Talbot, W.S. Gpr126 is essential for peripheral nerve development and myelination in mammals. Development 138, 2673–2680 (2011).
Glenn, T.D. & Talbot, W.S. Analysis of Gpr126 function defines distinct mechanisms controlling the initiation and maturation of myelin. Development 140, 3167–3175 (2013).
Mogha, A. et al. Gpr126 functions in Schwann cells to control differentiation and myelination via G-protein activation. J. Neurosci. 33, 17976–17985 (2013).
Ganfornina, M.D. et al. ApoD, a glia-derived apolipoprotein, is required for peripheral nerve functional integrity and a timely response to injury. Glia 58, 1320–1334 (2010).
Comley, L.H. et al. ApoE isoform–specific regulation of regeneration in the peripheral nervous system. Hum. Mol. Genet. 20, 2406–2421 (2011).
Michailov, G.V. et al. Axonal neuregulin-1 regulates myelin sheath thickness. Science 304, 700–703 (2004).
Eguchi, N. et al. Lack of tactile pain (allodynia) in lipocalin-type prostaglandin D synthase–deficient mice. Proc. Natl. Acad. Sci. USA 96, 726–730 (1999).
Trivedi, S.G. et al. Essential role for hematopoietic prostaglandin D2 synthase in the control of delayed type hypersensitivity. Proc. Natl. Acad. Sci. USA 103, 5179–5184 (2006).
Nelson, A.M. et al. Prostaglandin D2 inhibits wound-induced hair follicle neogenesis through the receptor, Gpr44. J. Invest. Dermatol. 133, 881–889 (2013).
Quattrini, A. et al. Beta 4 integrin and other Schwann cell markers in axonal neuropathy. Glia 17, 294–306 (1996).
Bolis, A. et al. Dlg1, Sec8 and Mtmr2 regulate membrane homeostasis in Schwann cell myelination. J. Neurosci. 29, 8858–8870 (2009).
Wrabetz, L. et al. A minimal human MBP promoter-lacZ transgene is appropriately regulated in developing brain and after optic enucleation, but not in shiverer mutant mice. J. Neurobiol. 34, 10–26 (1998).
Song, W.L., Lawson, J.A., Wang, M., Zou, H. & FitzGerald, G.A. Noninvasive assessment of the role of cyclooxygenases in cardiovascular health: a detailed HPLC/MS/MS method. Methods Enzymol. 433, 51–72 (2007).
Pellegatta, M. et al. alpha6beta1 and alpha7beta1 integrins are required in Schwann cells to sort axons. J. Neurosci. 33, 17995–18007 (2013).
Smyth, G. K. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 3, 3 (2004).
Acknowledgements
We thank V. Marzano and A. Urbani for shotgun mass spectrometry analyses, F. Clarelli for heat map and statistical analyses, G. Fitzgerald (University of Pennsylvania) for providing Gpr44−/− mice, P. Podini for excellent technical assistance with electron microscopy analyses, and P. Del Boccio for LC-MS/MS analyses. We are grateful to M. Buono, S. Previtali and A. Bolino for critical reading of the manuscript and suggestions, and L. Massimino for artwork. Part of this work was carried out in ALEMBIC, an advanced microscopy laboratory at the San Raffaele Scientific Institute. M.G.F. conducted this study in partial fulfillment for her Ph.D. in Molecular and Cellular Neuroscience, San Raffaele University. This study was supported by the Italian Minister of Health (award number GR08-35, C.T.), the European Marie Curie Reintegration Grant (award number IRG 239430-2008, C.T.) and the Agence Nationale pour la Recherche (ANR blanc programme, B.B.B.). C.T. is also supported by Italian Fondazione Italiana Sclerosi Multipla.
Author information
Authors and Affiliations
Contributions
A.T. designed and conducted the majority of the experiments. M.G.F., V.A. and A.L. contributed to in vitro and biochemical studies. P.B. and F.M.B. performed expression studies. G.D. and A.Q. performed morphological and ultrastructural analyses. Y.U. and B.B.-B. provided transgenic lines and provided input. D.P. and P.S. performed the MS/MS-LC analyses. C.T. designed the experimental plan, supervised the project and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
C.T. and A.T. submitted a patent on July 1 2014 (PCT/EP2014/063995) based on the work described in this paper.
Integrated supplementary information
Supplementary Figure 1 Differentially expressed genes in the microarray analyses.
a) Heat map diagram showing the expression levels of the differentially expressed genes from the comparison between NRG1 ICD infected DRG neurons and not infected (Limma moderated t–test P = <0.001; fold change cut off > 1.5 or < 0.66 df=6). N = 4 different biological replicates in each experimental condition. b) Venn diagram showing the number of differentially expressed genes in the comparisons tested in the microarray analyses (P = <0.01; fold change cut–off: 2). N = 4 different biological replicates in each experimental condition. c) RT–PCR on mRNA prepared from DRG neurons not infected, infected with a lentivirus expressing NRG1 ICD or EGFP as control. L–PGDS is upregulated only in neurons overexpressing NRG1 ICD. N = 3 different independent mRNA preparations and analyses.
Supplementary Figure 2 L–PGDS protein is not retained in DRG neurons.
a) Representative western blotting analyses of DRG neurons lysates not infected or infected with NRG1 ICD or L–PGDS as control. Only in L–PGDS overexpressing cultures it is possible to detect L–PGDS protein. Actin serves as a loading control. N = 3 different independent experiments. b) Representative western blotting analyses of DRG conditioned media from not infected or L–PGDS infected neurons. 14 days after infection, cultures were grown in the presence of neurobasal media for additional 48 hours after which the media was tested for L–PGDS expression. L–PGDS is released in the conditioned media. N = 3 different independent experiments. For uncropped pictures of western blots, see Supplementary Figure 10. c) Ponceau loading control of the Western blot showed in Figure 3a. N = 3 different independent experiments. d) Ponceau loading control of the Western blot showed in Figure 3b. N = 3 different independent experiments. e) LC–MS/MS analyses show that PGD2 is not present in conditioned media of DRG neurons overexpressing EGFP. Chromatograms are representative of three different experiments. N = 3 conditioned media preparations.
Supplementary Figure 3 H–PGDS–/– are normally myelinated.
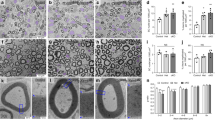
a) Representative immunofluorescence of two months old s–type, L–PGDS–/– and H–PGDS–/– sciatic nerve cross sections stained for H–PGDS (rhodamine). H–PDGS is expressed in wild–type and in L–PGDS–/– in similar amounts. Sections were also stained for neurofilament (fluorescein) and nuclei (DAPI). Lack of signal in H–PGDS–/– confirmed antibody specificity. N = 3 independent experiments. Bar: 50 μm. b–d) Morphological analyses of 1 month wild–type and H–PGDS–/– sciatic nerves. b) Electron micrographs and c) g–ratio analyses confirmed normal myelination in H–PGDS–/–. g–ratio as a function of axon diameter is similar in wild–type (red line) and H–PGDS–/– (black line) (t–test analysis; P = 0.4261; t=0.7964, df=662). The graph represents the g–ratio obtained from more than 250 myelinated axons. d) Distribution of myelinated fibers is similar in H–PGDS–/– and wild–type 1 month sciatic nerves. Fisher’s exact test; P = 0.4449 (total vs 1–2 μm), P = 0.5546 (total vs 2–3 μm), P = 0.2521 (total vs 3–4 μm), P = 0.1694 (total vs 4–5 μm), P = 1 (total vs 5–6 μm). Over 80 fibers for each genotype were counted. N = 3 mice per genotype. Bar: 2 μm. e–g) Morphological analyses of 6 month wild–type and H–PGDS–/– sciatic nerves. e) Electron micrographs and f) g–ratio analyses confirmed normal myelination in H–PGDS–/–. g–ratio as a function of axon diameter is similar in wild–type (red line) and H–PGDS–/– (black line) (t–test analysis; P = 0.6071; t=0.5725, df=388). The graph represents the g–ratio obtained from more than 150 myelinated axons. g) Distribution of myelinated fibers is similar in H–PGDS–/– and wild–type 6 month sciatic nerves. Fisher’s exact test; P = 0.2325 (total vs 1–2 μm), P = 0.8385 (total vs 2–3 μm), P = 0.811 (total vs 3–4 μm), P = 0.1056 (total vs 4–5 μm), P = 0.4431 (total vs 5–6 μm), P = 1 (total vs 6–7 μm), P = 0.2243 (total vs 7–8 μm), P = 1 (total vs >8 μm). Over 60 fibers for each genotype were counted. N = 3 mice per genotype. Bar: 2 μm.
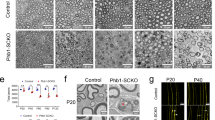
Supplementary Figure 4 In vitro H–PGDS–/–;L–PGDS–/– neurons are hypomyelinated.
a–b) Representative electron microscopy analyses of wild–type (a) and H–PGDS–/–;L–PGDS–/– (b) mouse DRG neurons seeded with rat wild–type Schwann cells and maintained in myelinating conditions for 9 days. Null cocultures are hypomyelinated, and when formed myelin is aberrant (arrow). They also present several naked axons (asterisks), unlike control cultures. N = 9 different independent coculture experiments. Bar: 1 μm c) High power images showing the significant difference in myelin sheath thickness in null cocultures as compared to that of wild–type axons of similar caliber. N = 9 different independent coculture experiments. Bar: 500 nm.
Supplementary Figure 5 The sorting process is normal in H–PGDS–/–;L–PGDS–/– and saphenous nerves are hypomyelinated.
a) Morphological analyses of P2 wild–type and H–PGDS–/–;L–PGDS–/– sciatic nerves. H–PGDS–/–;L–PGDS–/– are significantly hypomyelinated, but not impaired in axonal sorting. Bottom panel shows a significantly hypomyelinated axon a feature common to many null fibers. N = 3 mice per genotype. Bar: 2 μm, top panels, 1 μm bottom panels. b–d) Morphological analyses of 8 month wild–type and H–PGDS–/–;L–PGDS–/– saphenous nerves. b) Electron micrographs and c) g–ratio analyses confirmed hypomyelination in H–PGDS–/–;L–PGDS–/– saphenous nerves. g–ratio as a function of axon diameter is similar in wild–type (red line) and H–PGDS–/–;L–PGDS–/– (black line) (t–test analysis; P = <0.0001; t=8.448, df=662). The graph represent the g–ratio obtained from more than 300 myelinated axons. d) Distribution of myelinated fibers is similar in H–PGDS–/–;L–PGDS–/– and wild–type saphenous nerves. Fisher’s exact test; P = 1 (total vs 1–2 μm), P = 0.7667 (total vs 2–3 μm), P = 0.9181 (total vs 3–4 μm), P = 0.6889 (total vs 4–5 μm), P = 0.6445 (total vs 5–6 μm), P = 0.4917 (total vs 6–7 μm). Over 100 fibers for each genotype were counted. N = 3 mice per genotype. Bar: 2 μm.
Supplementary Figure 6 The number of myelinated axons and Remak fibers are normal in H–PGDS–/–;L–PGDS–/–.
a) The total number of myelinated axons per area is similar in motor roots of 1 month old wild–type and H–PGDS–/–;L–PGDS–/–. Myelinated fibers were counted over the entire root (n=3). (t–test analysis; P = 0.0517; t=2.744, df=4). N = 3 mice per genotype. Error bars represent means ± s.e.m. b) The total number of myelinated axons per area is similar in sensory roots of 1 month old wild–type and H–PGDS–/–;L–PGDS–/– mice. Myelinated fibers were counted over the entire root (n=3) (t–test analysis; P = 0.051; t=2.756, df=4). N = 3 mice per genotype. Error bars represent means ± s.e.m. c) The total number of myelinated axons per area is similar in motor roots of 8 months old wild–type and H–PGDS–/–;L–PGDS–/– mice. Myelinated fibers were counted over the entire root (n=3) (t–test analysis; P = 0.9689; t=0.04154, df=4). N = 3 mice per genotype. Error bars represent means ± s.e.m. d) The total number of myelinated axons per area is similar in sensory roots of 8 months old wild–type and H–PGDS–/–;L–PGDS–/– mice. Myelinated fibers were counted over the entire root (n=3) (t–test analysis; P = 0.1571; t=1.739, df=4). N = 3 mice per genotype. Error bars represent means ± s.e.m. e) The total number of myelinated axons per area is similar in 9 months old sciatic nerves of wild–type and H–PGDS–/–;L–PGDS–/– mice. Myelinated fibers were counted over the entire sciatic nerve cross sections (t–test analysis; P = 0.6075; t=0.5566, df=4). N = 3 mice per genotype. Error bars represent means ± s.e.m. f–g) The number of axons per Remak bundle in wild–type and H–PGDS–/–;L–PGDS–/– sciatic nerves were binned into separate groups and are shown as a percentage of the total axons. Over 120 bundles for each genotype were counted. The number of axons/bundle is similar in 1 month (e) and 9 months old mice (f). Fisher’s exact test 1 month old mice; P = 0.5021 (total vs 1–5), P = 0.9063 (total vs 5–10), P = 0.6173 (total vs 11–15 μm), P = 0.2234 (total vs 16–20 μm), P = 1 (total vs 21–25 μm), P = 0.8052 (total vs 26–30 μm), P = 0.2923 (total vs 31–35 μm), P = 0.0513 (total vs 36–40 μm), P = 0.251 (total vs 41–45 μm). Fisher’s exact test 9 months old mice; P = 0.6537 (total vs 1–5), P = 0.2255 (total vs 5–10), P = 0.3149 (total vs 11–15 μm), P = 1 (total vs 16–20 μm), P = 0.5191 (total vs 21–25 μm), P = 0.3447 (total vs 26–30 μm), P = 1 (total vs 31–35 μm), P = 0.3539 (total vs 36–40 μm), P = 0.2685 (total vs 41–45 μm), P = 0.359 (total vs > 50). N = 3 mice per genotype.
Supplementary Figure 7 AT–56 effects on Schwann cells and neuronal survival, axon–Schwann cell interaction and myelination.
a) Graph showing the dose dependent effects of AT–56 treatment on Schwann cell survival, determined as percentage of Caspase 3+ cells over the total number of cells (3 coverslip/condition; 3 different experiments). 20 μM AT–56 does not alter Schwann cells survival. Error bars represent means ± s.e.m. (t–test analysis: untreated – 20 μM P = 0.1138; t=1.807, df=4; untreated – 50 μM P < 0.0001; t=7.028, df=7). N = 3 different experiments. b) Graph showing the dose dependent effects of AT–56 treatment on myelinating Schwann cell–DRG neuronal coculture, determined as number of MBP+ segments per field (3 coverslip/condition; 3 different experiments). Increased concentration of AT–56 impairs myelination. Error bars represent means ± s.e.m. (t–test analysis: untreated – 1 μM P = 0.3866; t=0.8806, df=26; untreated – 10 μM *** P <0.0001; t=5.651, df=31; untreated – 25 μM *** P <0.0001; t=6.736, df=22). N = 3 different experiments. c) Graph showing the dose dependent effects of AT–56 treatment on Schwann cell–DRG neuronal coculture survival. We counted the effects of AT–56 on Schwann cells and express it as percentage of Caspase 3+ Schwann cells over the total number of cells (3 coverslip/condition; 3 different experiments). 25 μM AT–56 does not alter Schwann cells survival. (t–test analysis: untreated – 25 μM p = 0.3874; t=0.9318 df=6). Error bars represent means ± s.e.m. N = 3 different experiments. d) Organotypic mouse DRG explants were maintained in the presence of 25 μM AT–56 for 7 days then stained for S100 (rhodamine) and β–tubulin (fluorescein). AT–56 treatment does not impair axon – Schwann cell association. N = 3 different coculture experiments. Bar: 50 μm.
Supplementary Figure 8 PGD2 receptors expression and their role in myelination.
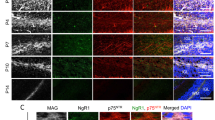
a) qRT–PCR analyses in mRNA prepared from not infected or NRG1 ICD infected DRG neurons, primary rat Schwann cells and skeletal muscle, which serves as a control. Ptgdr expression is comparable among all samples. Expression levels were normalized to Gapdh levels. Data were analyzed with the CFX Manager SoftwareTM from Biorad on three mRNA different preparations. Error bars represent means ± s.d. (One–way ANOVA; F=10.77; P = 0.0648 SC – Not Inf; P = 0.2853 SC – ICD and P = 0.04 Not Inf – ICD). N = 3 different independent mRNA preparations and analyses. b) Cocultures of mouse organotypic Schwann cells DRG neurons infected with Gpr44 specific shRNAs and scramble (shscr) lentiviruses were maintained in myelinating conditions for 7 d, fixed and stained for MBP (rhodamine) and neurofilament (fluorescein). Less myelin segments are evident in shGpr44–infected cultures. Shown images are representative of three different independent experiments. N = 3 different coculture experiments. Bar: 100 μm.
Supplementary Figure 9 PGD2 does not modulate AKT and MAPK pathways.
PGD2 does not activate AKT–1 and MAPK pathways. Rat primary Schwann were grown to confluence and then starved for 16 hours. Schwann cells were then treated with 2.5 ng/ml NRG1β1, 10 nM Cyclosporin A, 100 nM EtOH, 100 nM PGD2, 2.5 μM forskolin, 100 nM PGD2 together with 2.5 μM forskolin. Schwann cells lysates were tested by western blotting analyses for (a) AKT–1 phosphorylation (ser 473) and total AKT–1 levels and (b) p44/p42 phosphorylation and total p44/p42 levels. Shown images are representative of three different independent experiments. N = 3 different independent experiments. For uncropped pictures of western blots, see Supplementary Figure 10. c) Model of L–PGDS/GPR44 activity. NRG1 ICD, upon generation, translocates into the nucleus to specifically activate L–PGDS mRNA expression. L–PGDS protein is then released in the extracellular milieu where is enzymatically active. PGD2 binds to and activates glial Gpr44 resulting in dephosphorylation and nuclear translocation of Nfatc4 to promote myelination.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 and Supplementary Tables 2 and 3 (PDF 10177 kb)
Supplementary Methods Checklist
(PDF 616 kb)
Supplementary Table 1: List of genes upregulated in the Illumina analyses.
List of genes whose mRNA were upregulated with a FC >2.0 and a P = <0.01 in DRG ICD infected neurons versus not infected, corrected for genes upregulated in EGFP infected neurons. N = 4 different independent RNA preparations and analyses. (XLS 34 kb)
Rights and permissions
About this article
Cite this article
Trimarco, A., Forese, M., Alfieri, V. et al. Prostaglandin D2 synthase/GPR44: a signaling axis in PNS myelination. Nat Neurosci 17, 1682–1692 (2014). https://doi.org/10.1038/nn.3857
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3857
This article is cited by
-
The Schwann cell-specific G-protein Gαo (Gnao1) is a cell-intrinsic controller contributing to the regulation of myelination in peripheral nerve system
Acta Neuropathologica Communications (2024)
-
LXR agonist modifies neuronal lipid homeostasis and decreases PGD2 in the dorsal root ganglia in western diet-fed mice
Scientific Reports (2022)
-
Lipocalin-2-Mediated Insufficient Oligodendrocyte Progenitor Cell Remyelination for White Matter Injury After Subarachnoid Hemorrhage via SCL22A17 Receptor/Early Growth Response Protein 1 Signaling
Neuroscience Bulletin (2022)
-
Quantitative proteomics revealed extensive microenvironmental changes after stem cell transplantation in ischemic stroke
Frontiers of Medicine (2022)
-
A single-cell survey unveils cellular heterogeneity and sensitive responses in mouse cortices induced by oral exposure to triphenyl phosphate
Archives of Toxicology (2022)