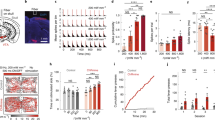
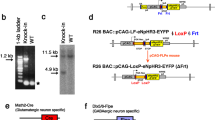
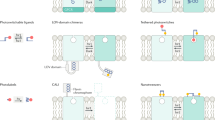
Abstract
Optogenetic inhibition of the electrical activity of neurons enables the causal assessment of their contributions to brain functions. Red light penetrates deeper into tissue than other visible wavelengths. We present a red-shifted cruxhalorhodopsin, Jaws, derived from Haloarcula (Halobacterium) salinarum (strain Shark) and engineered to result in red light–induced photocurrents three times those of earlier silencers. Jaws exhibits robust inhibition of sensory-evoked neural activity in the cortex and results in strong light responses when used in retinas of retinitis pigmentosa model mice. We also demonstrate that Jaws can noninvasively mediate transcranial optical inhibition of neurons deep in the brains of awake mice. The noninvasive optogenetic inhibition opened up by Jaws enables a variety of important neuroscience experiments and offers a powerful general-use chloride pump for basic and applied neuroscience.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Chow, B.Y. et al. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature 463, 98–102 (2010).
Gradinaru, V. et al. Molecular and cellular approaches for diversifying and extending optogenetics. Cell 141, 154–165 (2010).
Han, X. et al. A high-light sensitivity optical neural silencer: development and application to optogenetic control of non-human primate cortex. Front. Syst. Neurosci. 5, 18 (2011).
Gradinaru, V., Thompson, K.R. & Deisseroth, K. eNpHR: a Natronomonas halorhodopsin enhanced for optogenetic applications. Brain Cell Biol. 36, 129–139 (2008).
Ibañez-Tallon, I. et al. Tethering naturally occurring peptide toxins for cell-autonomous modulation of ion channels and receptors in vivo. Neuron 43, 305–311 (2004).
Armbruster, B.N., Li, X., Pausch, M.H., Herlitze, S. & Roth, B.L. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl. Acad. Sci. USA 104, 5163–5168 (2007).
Kramer, R.H., Mourot, A. & Adesnik, H. Optogenetic pharmacology for control of native neuronal signaling proteins. Nat. Neurosci. 16, 816–823 (2013).
Polikov, V.S., Tresco, P.A. & Reichert, W.M. Response of brain tissue to chronically implanted neural electrodes. J. Neurosci. Methods 148, 1–18 (2005).
Nimmerjahn, A., Kirchhoff, F. & Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314–1318 (2005).
Xu, H.T., Pan, F., Yang, G. & Gan, W.B. Choice of cranial window type for in vivo imaging affects dendritic spine turnover in the cortex. Nat. Neurosci. 10, 549–551 (2007).
Drew, P.J. et al. Chronic optical access through a polished and reinforced thinned skull. Nat. Methods 7, 981–984 (2010).
Huber, D. et al. Sparse optical microstimulation in barrel cortex drives learned behaviour in freely moving mice. Nature 451, 61–64 (2008).
Scott, N.A. & Murphy, T.H. Hemodynamic responses evoked by neuronal stimulation via channelrhodopsin-2 can be independent of intracortical glutamatergic synaptic transmission. PLoS ONE 7, e29859 (2012).
Hira, R. et al. Transcranial optogenetic stimulation for functional mapping of the motor cortex. J. Neurosci. Methods 179, 258–263 (2009).
Li, X. et al. Fast noninvasive activation and inhibition of neural and network activity by vertebrate rhodopsin and green algae channelrhodopsin. Proc. Natl. Acad. Sci. USA 102, 17816–17821 (2005).
Lin, J.Y., Knutsen, P.M., Muller, A., Kleinfeld, D. & Tsien, R.Y. ReaChR: a red-shifted variant of channelrhodopsin enables deep transcranial optogenetic excitation. Nat. Neurosci. 16, 1499–1508 (2013).
Otomo, J., Tomioka, H. & Sasabe, H. Bacterial rhodopsins of newly isolated halobacteria. J. Gen. Microbiol. 138, 1027–1037 (1992).
Mattis, J. et al. Principles for applying optogenetic tools derived from direct comparative analysis of microbial opsins. Nat. Methods 9, 159–172 (2012).
Hackett, N.R., Stern, L.J., Chao, B.H., Kronis, K.A. & Khorana, H.G. Structure-function studies on bacteriorhodopsin. V. Effects of amino acid substitutions in the putative helix F. J. Biol. Chem. 262, 9277–9284 (1987).
Rüdiger, M. & Oesterhelt, D. Specific arginine and threonine residues control anion binding and transport in the light-driven chloride pump halorhodopsin. EMBO J. 16, 3813–3821 (1997).
Ma, D. et al. Role of ER export signals in controlling surface potassium channel numbers. Science 291, 316–319 (2001).
Hofherr, A., Fakler, B. & Klocker, N. Selective Golgi export of Kir2.1 controls the stoichiometry of functional Kir2.x channel heteromers. J. Cell Sci. 118, 1935–1943 (2005).
Sung, C.H. & Chuang, J.Z. The cell biology of vision. J. Cell Biol. 190, 953–963 (2010).
Busskamp, V. et al. Genetic reactivation of cone photoreceptors restores visual responses in retinitis pigmentosa. Science 329, 413–417 (2010).
Farber, D.B., Flannery, J.G. & Bowes-Rickman, C. The rd mouse story: seventy years of research on an animal model of inherited retinal degeneration. Prog. Retin. Eye Res. 13, 31–64 (1994).
Busskamp, V. & Roska, B. Optogenetic approaches to restoring visual function in retinitis pigmentosa. Curr. Opin. Neurobiol. 21, 942–946 (2011).
Madisen, L. et al. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat. Neurosci. 15, 793–802 (2012).
Arrenberg, A.B., Del Bene, F. & Baier, H. Optical control of zebrafish behavior with halorhodopsin. Proc. Natl. Acad. Sci. USA 106, 17968–17973 (2009).
Tsunematsu, T. et al. Acute optogenetic silencing of orexin/hypocretin neurons induces slow-wave sleep in mice. J. Neurosci. 31, 10529–10539 (2011).
Tønnesen, J., Sorensen, A.T., Deisseroth, K., Lundberg, C. & Kokaia, M. Optogenetic control of epileptiform activity. Proc. Natl. Acad. Sci. USA 106, 12162–12167 (2009).
Znamenskiy, P. & Zador, A.M. Corticostriatal neurons in auditory cortex drive decisions during auditory discrimination. Nature 497, 482–485 (2013).
Cardin, J.A. Dissecting local circuits in vivo: integrated optogenetic and electrophysiology approaches for exploring inhibitory regulation of cortical activity. J. Physiol. Paris 106, 104–111 (2012).
Raimondo, J.V., Kay, L., Ellender, T.J. & Akerman, C.J. Optogenetic silencing strategies differ in their effects on inhibitory synaptic transmission. Nat. Neurosci. 15, 1102–1104 (2012).
Tye, K.M. et al. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature 471, 358–362 (2011).
Yizhar, O., Fenno, L.E., Davidson, T.J., Mogri, M. & Deisseroth, K. Optogenetics in neural systems. Neuron 71, 9–34 (2011).
Al-Juboori, S.I. et al. Light scattering properties vary across different regions of the adult mouse brain. PLoS ONE 8, e67626 (2013).
Giller, C.A. et al. Validation of a near-infrared probe for detection of thin intracranial white matter structures. J. Neurosurg. 98, 1299–1306 (2003).
Jacobs, G.H., Williams, G.A., Cahill, H. & Nathans, J. Emergence of novel color vision in mice engineered to express a human cone photopigment. Science 315, 1723–1725 (2007).
Naarendorp, F. et al. Dark light, rod saturation, and the absolute and incremental sensitivity of mouse cone vision. J. Neurosci. 30, 12495–12507 (2010).
Anikeeva, P. et al. Optetrode: a multichannel readout for optogenetic control in freely moving mice. Nat. Neurosci. 15, 163–170 (2012).
Lee, S.-H. et al. Activation of specific interneurons improves V1 feature selectivity and visual perception. Nature 488, 379–383 (2012).
Grutzendler, J., Kasthuri, N. & Gan, W.B. Long-term dendritic spine stability in the adult cortex. Nature 420, 812–816 (2002).
Yoder, E.J. & Kleinfeld, D. Cortical imaging through the intact mouse skull using two-photon excitation laser scanning microscopy. Microsc. Res. Tech. 56, 304–305 (2002).
Berényi, A., Belluscio, M., Mao, D. & Buzsaki, G. Closed-loop control of epilepsy by transcranial electrical stimulation. Science 337, 735–737 (2012).
Trachtenberg, J.T. et al. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature 420, 788–794 (2002).
Jazayeri, M., Lindbloom-Brown, Z. & Horwitz, G.D. Saccadic eye movements evoked by optogenetic activation of primate V1. Nat. Neurosci. 15, 1368–1370 (2012).
Ohayon, S., Grimaldi, P., Schweers, N. & Tsao, D.Y. Saccade modulation by optical and electrical stimulation in the macaque frontal eye field. J. Neurosci. 33, 16684–16697 (2013).
Ye, H., Daoud-El Baba, M., Peng, R.W. & Fussenegger, M. A synthetic optogenetic transcription device enhances blood-glucose homeostasis in mice. Science 332, 1565–1568 (2011).
Menzler, J. & Zeck, G. Network oscillations in rod-degenerated mouse retinas. J. Neurosci. 31, 2280–2291 (2011).
Paxinos, G. & Franklin, K.B.J. The Mouse Brain in Stereotaxic Coordinates, compact 2nd edn. (Elsevier Academic, Amsterdam and Boston, 2004).
Klapoetke, N.C. et al. Independent optical excitation of distinct neural populations. Nat. Methods 11, 338–346 (2014).
Grieger, J.C., Choi, V.W. & Samulski, R.J. Production and characterization of adeno-associated viral vectors. Nat. Protoc. 1, 1412–1428 (2006).
Hippenmeyer, S. et al. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol. 3, e159 (2005).
Kodandaramaiah, S.B., Franzesi, G.T., Chow, B.Y., Boyden, E.S. & Forest, C.R. Automated whole-cell patch-clamp electrophysiology of neurons in vivo. Nat. Methods 9, 585–587 (2012).
Cardin, J.A. et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 459, 663–667 (2009).
Binzoni, T., Leung, T.S., Gandjbakhche, A.H., Rufenacht, D. & Delpy, D.T. The use of the Henyey-Greenstein phase function in Monte Carlo simulations in biomedical optics. Phys. Med. Biol. 51, N313–N322 (2006).
Wang, L., Jacques, S.L. & Zheng, L. MCML–Monte Carlo modeling of light transport in multi-layered tissues. Comput. Methods Programs Biomed. 47, 131–146 (1995).
Yaroslavsky, A.N. et al. Optical properties of selected native and coagulated human brain tissues in vitro in the visible and near infrared spectral range. Phys. Med. Biol. 47, 2059–2073 (2002).
Hatazawa, J. et al. Regional cerebral blood flow, blood volume, oxygen extraction fraction, and oxygen utilization rate in normal volunteers measured by the autoradiographic technique and the single breath inhalation method. Ann. Nucl. Med. 9, 15–21 (1995).
Kreiss, P., Bettan, M., Crouzet, J. & Scherman, D. Erythropoietin secretion and physiological effect in mouse after intramuscular plasmid DNA electrotransfer. J. Gene Med. 1, 245–250 (1999).
Roggan, A., Friebel, M., Dorschel, K., Hahn, A. & Muller, G. Optical properties of circulating human blood in the wavelength range 400–2500 nm. J. Biomed. Opt. 4, 36–46 (1999).
Bashkatov, A.N., Genina, E.A., Kochubey, V.I. & Tuchin, V.V. Optical properties of human cranial bone in the spectral range from 800 to 2000 nm — art. no. 616310. Saratov Fall Meeting 2005: Optical Technologies in Biophysics and Medicine VII 6163, 16310 (2006).
Ugryumova, N., Matcher, S.J. & Attenburrow, D.P. Measurement of bone mineral density via light scattering. Phys. Med. Biol. 49, 469–483 (2004).
Tsubota, T., Ohashi, Y., Tamura, K., Sato, A. & Miyashita, Y. Optogenetic manipulation of cerebellar Purkinje cell activity in vivo. PLoS ONE 6, e22400 (2011).
Boyden, E.S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268 (2005).
Han, X. & Boyden, E.S. Multiple-color optical activation, silencing, and desynchronization of neural activity, with single-spike temporal resolution. PLoS ONE 2, e299 (2007).
Acknowledgements
We thank J. Juettner for help making AAV, and Y.K. Cho, D. Schmidt, F. Chen, A. Beyeler, J.M. Zhuo and R.E. Kohman for advice and discussion. A.S.C. acknowledges the Janet and Sheldon Razin ′59 Fellowship of the Massachusetts Institute of Technology (MIT) McGovern Institute. E.S.B. acknowledges Jerry and Marge Burnett, the US Defense Advanced Research Projects Agency Living Foundries Program HR0011-12-C-0068, Harvard/MIT Joint Grants Program in Basic Neuroscience, Human Frontiers Science Program, Institution of Engineering and Technology A F Harvey Prize, MIT McGovern Institute and McGovern Institute Neurotechnology (MINT) Program, MIT Media Lab, New York Stem Cell Foundation-Robertson Investigator Award, US National Institutes of Health (NIH) Director's New Innovator award 1DP2OD002002, NIH EUREKA award 1R01NS075421, NIH grants 1R01DA029639, 1RC1MH088182 and 1R01NS067199, US National Science Foundation (NSF) CAREER award CBET 1053233 and NSF grants EFRI0835878 and DMS0848804, the Skolkovo Institute of Science and Technology, a Society for Neuroscience Research Award for Innovation in Neuroscience (RAIN) and the Wallace H. Coulter Foundation. M.L.M. acknowledges funding from NSF DGE 1122492. J.A.C. acknowledges funding from the Whitehall Foundation, the Klingenstein Foundation, the Swebelius Family Trust, the Simons Foundation, an Alfred P. Sloan Fellowship, a NARSAD Young Investigator Award, a Smith Family Award for Excellence in Biomedical Research, NIH R00 EY018407, NIH R01 EY022951 and NIH R01 MH102365. V.B. acknowledges Human Frontier Science Program, Swiss National Science Foundation and Volkswagen Foundation fellowships. B.R. acknowledges the Gebert-Ruf Foundation, SNSF, European Research Council, and European Union SEEBETTER, TREATRUSH, OPTONEURO and 3X3D Imaging grants. X.H. acknowledges funding from an NIH Director's New Innovator Award (1DP2NS082126), the NINDS (1R01NS087950, 1R21NS078660, 1R01NS081716), NIMH (5R00MH085944), Pew Foundation, Alfred P. Sloan Foundation, Michael J. Fox Foundation, and Brain and Research Foundation. Y.L. acknowledges funding from NIH RO1 MH091220-01. B.Y.C. acknowledges funding from US Defense Advanced Research Projects Agency Living Foundries, the US National Science Foundation Biophotonics and the Brain Research Foundation. K.M.T. acknowledges funding from the Whitehall Foundation, Klingenstein Foundation, JPB Foundation, PIIF Funding, R01-MH102441-01 (NIMH) and DP2-OD-017366-01. G.A.C.M. was supported by the Simons Center for the Social Brain.
Author information
Authors and Affiliations
Contributions
A.S.C. and E.S.B. coordinated all experiments and data analysis. A.S.C. designed and developed Jaws and cloned all constructs. A.S.C. performed in vivo glass pipette extracellular recordings and in vitro electrophysiology. M.L.M. and J.A.C. performed in vivo tetrode extracellular recordings. V.B. performed in vivo multielectrode array recordings. A.S.C., G.A.C.M., A.T.S. and A.Y. performed slice electrophysiology. A.S.C., M.L.M., G.A.C.M., A.T.S., J.A.C., V.B. and M.O. performed in vivo viral injections. A.S.C., M.L.M., V.B., G.A.C.M., A.T.S., S.B.R. and M.O. performed histological processing and fluorescence imaging. S.B.K. and C.R.F. designed or performed autopatch experiments. A.S.C. and N.C.K. performed transfections, cell culture and in vitro viral infections. M.A.H. conducted Monte Carlo modeling. L.C.A. carried out light propagation measurements. R.C.B. and B.D.A. carried out X-ray scans to measure mouse skull thicknesses for the Monte Carlo model. A.S.C., M.L.M., V.B., G.A.C.M., A.T.S., B.Y.C., X.H., J.A.C., B.R. and E.S.B. contributed to study design and data interpretation. J.A.C., B.R., K.M.T., Y.L. and E.S.B. supervised all aspects of the work. A.S.C. and E.S.B. wrote the paper with contributions from the other authors.
Corresponding author
Ethics declarations
Competing interests
A.S.C., E.S.B., N.C.K., B.Y.C. and X.H. have filed a patent on Jaws (owned by the Massachusetts Institute of Technology).
Integrated supplementary information
Supplementary Figure 1 Red versus green light propagation.
(a) Monte Carlo models of green (left, 532 nm) vs. red (right, 632 nm) transcranial light propagation into the brain when delivered via a 1500 μm fiber placed on the surface of the skull; the skull is interposed between fiber surface and brain, with skull-brain interface indicated by dotted line. (b) Monte Carlo model of green (left, 532 nm) vs. red (right, 632 nm) transdural light propagation when delivered via a 1500 μm fiber placed on the surface of the brain. (c) Relative fluence of green (left, 532 nm) vs. red (right, 635 nm) light measured in the anesthetized mouse brain when delivered via a 1500 μm fiber placed on the surface of the brain (n = 4 mice for green light; n = 5 mice for red light). Values are means ± standard deviation.
Supplementary Figure 2 Characterization of cruxhalorhodopsin class halorhodopsins.
(a) Cruxhalorhodopsin phylogeny tree. Scale bar indicates number of amino acid substitutions per site. (b) Members of the cruxhalorhodopsin class have uniquely red-shifted spectra compared to the N. pharaonis halorhodopsin (halo/NpHR), as reflected by red-green photocurrent ratios in cultured cortical neurons. Regressed lines are shown for each opsin, indicating distinct color shifts. (5 mW/mm2 at 543 or 632 nm). (c) Photocurrents of cruxhalorhodopsin class members as a function of red light irradiance (632 nm; n = 6 cells for each opsin). Values are means ± standard error. All measurements were taken in primary hippocampal neuron culture.
Supplementary Figure 3 Molecular analysis and physiological characterization of Jaws variants.
(a) Sequence alignment of Halo57, the H. salinarum strain shark cruxhalorhodopsin, with the N. pharaonis halorhodopsin, demonstrating < 60% sequence homology; blue residues denote sequence divergence, and grey residues, conservation. (b) Schematic of Halo57, Jaws, and Jaws-ER2 proteins. Black denotes the Halo57 protein scaffold, red indicates the K200R and W214F point mutations, green indicates the C terminal GFP fusion, and KGC and ER2 respectively refer to endoplasmic reticulum forward transport and Golgi export sequences from the potassium channel Kir2.1. (c-d) The K200R W214F mutation boosts Halo57 photocurrents (n = 3 cells for wildtype, n = 6 cells for K200R W214F mutant), but the homologous K215R W229F mutations cause no effect in eNpHR3.0 at 5 mW/mm2 (c) or across a range of 632 nm light powers (d; n = 3 cells for both wildtype and mutant eNpHR3.0). (e) Current-voltage relationship for light-activated Jaws photocurrents (n = 5 cells; 632 nm, 5 mW/mm2). Error bars are smaller than the symbols plotted. (f) Jaws photocurrent is dependent on [Cl-] in the extracellular bath solution (n = 3-5 cells; 632 nm, 5 mW/mm2). All measurements were taken in HEK293FT cells; values are mean ± standard error.
Supplementary Figure 4 Side-by-side comparison of different hyperpolarizing opsins.
(a) Representative phase contrast (left), tdTomato (middle) and GFP (right) images of a tdTomato and opsin-GFP fusion transfected neuron in culture. Scale bar is 50 μm. (b-c) Pooled tdTomato fluorescence (b) as well as plotted vs. photocurrent density (c) for ArchT (left), Jaws (middle), and eNpHR3.0 (right). Photocurrents were measured at 5 mW/mm2; 632 nm for Jaws (n = 26 cells) and eNpHR3.0 (n = 21 cells), and at 543 nm for ArchT (n = 30 cells). (d) Photocurrents for Jaws (n = 26 cells), eNpHR3.0 (n = 21 cells) and ArchT (n = 30 cells) as a function of red light irradiance (632 nm) as measured in transfected neuron culture. (e-f) Comparison of hyperpolarizing opsin on-kinetics and off-kinetics using red or green illumination for Jaws (n = 24 cells), eNpHR3.0 (n = 20 cells) or ArchT (n = 29 cells). (g) Neuron properties upon opsin expression in culture for Jaws (n = 33 cells), ArchT (n = 36 cells), and eNpHR3.0 (n = 29 cells) as compared to untransfected cells (n = 15), including cell membrane capacitance, holding current when held at -65 mV, resting potential, and cell input resistance. Values are means ± standard error. Statistics for panels d and h: ** P < 0.01, *** P < 0.001. Panel d was an ANOVA with a Newman-Keuls post hoc test, panel g was an ANOVA with Dunnett's post hoc test using untransfected neurons as the reference.
Supplementary Figure 5 Light responses mediated by cone expression and illumination of Halo57 (K200R W214F) in murine retinitis pigmentosa retinas.
(a) Comparison of mean spiking for Halo57 (K200R W214F) mutant (n = 16) in ganglion cells downstream from opsin-expressing neurons (9.6 x 1017 photons cm-2 s-1 at 600 nm), using AAV with the mCAR promoter and serotype 8, ∼ 40 days post infection. (b) Confocal fluorescence images of Jaws-GFP (left) and eNpHR3.0-expressing (right) f-RD retinas. Scale bars 20 μm. (c) Retinal ganglion cell spike rates vs. red (left), green (middle), and blue (right) irradiances, comparing Halo57 (K200R W214F) vs. eNpHR. (d) Comparison of retinal ganglion cell spiking under red, green, and blue illumination for eNpHR (n = 21 cells), Halo57 (n = 14 cells), Halo57 (K200R W214F) (n = 16 cells), Jaws (n = 27 cells), ArchT (n = 30 cells) or Mac (n = 13 cells) expressed in mouse cone cells (light intensity was 6.7 x 1017 photons cm-2 s-1 at 470 nm, 1.2 x 1018 photons cm-2 s-1 at 550 nm, and 9.6 x 1017 photons cm-2 s-1 at 600 nm). Values are means ± standard error.
Supplementary Figure 6 Jaws shuts down fast-firing interneurons in visual cortex.
(a) Post-stimulus time histograms for a putative fast-spiking interneuron in the visual cortex of an anesthetized PV-Cre mouse injected with AAV5-FLEX-Jaws virus (35 mW/mm2 at 593 nm using a 200 μm fiber) undergoing visual stimulation (left) and Jaws-mediated inhibition of a visually evoked response (right). (b) Post-stimulus time histogram for a standard step light pulse (black line) versus ramped illumination (yellow line), for two spontaneously firing visual cortex neurons.
Supplementary Figure 7 Ex vivo characterization of Jaws in acute motor cortex slice.
(a) Jaws photocurrents (left) and photocurrent densities (right), measured as a function of red light irradiance, were the same at 4 and 6 weeks post-injection in acute slice (n = 8 cells for each timepoint). (b) Jaws yellow and red light photocurrents (n = 16 cells, 5 mW/mm2, 593 or 632 nm). (c) Comparison of Jaws on-kinetics (left) and off-kinetics (right) using red or yellow illumination (n = 16 cells for each, 5 mW/mm2 593 or 632 nm). (d) Neuron properties upon Jaws expression at 4 (n = 9 cells) or 6 weeks (n = 10 cells) post-injection in acute cortical slice as compared against non-opsin-expressing neurons (n = 5 cells at 4 weeks, n = 3 cells at 6 weeks), including cell membrane capacitance (left), resting potential (middle), and cell input resistance (right). Values are means ± standard error. Statistics for panels d: * P < 0.05. Panel d was an ANOVA with Dunnett's post hoc test using non-opsin expressing neurons as the reference.
Supplementary Figure 8 Demonstration of Jaws functionality in awake mouse cortex, using invasive 200-μm fibers.
(a-b) Gene schematic (a) and corresponding representative glass pipette extracellular recording (b) of different Jaws variants expressed in cortical neurons 6 weeks post-injection in awake mice undergoing red or yellow light illumination (637 or 593 nm, ∼ 130 mW/mm2 out the fiber tip). (c) Light-induced hyperpolarization of a Jaws-expressing neuron patched in the motor cortex of an anesthetized mouse (AAV8-CAG-Jaws; 635 nm; ∼ 130 mW/mm2 out the fiber tip terminating ∼ 500 μm above the electrode tip). (d) Comparison of different Jaws variants 1-3 months post-injection, as measured by suppression of spontaneous firing, change in firing 5-seconds post-illumination, inhibition latency, and recovery latency (n = 14 units for AAV8-CaMKII-Jaws, n = 17 units for AAV8-hSyn-Jaws, n = 6 units for AAV8-hSyn-Jaws-ER2). (e) Spike rasters recorded from a representative neuron (top), and population average (bottom; n = 31 units) of instantaneous firing rate in neurons showing any degree of light-induced suppression, recorded in awake headfixed mice 4-8 weeks after injection of AAV8 encoding Jaws under either the CaMKII (n = 14 units) or synapsin promoter (n = 17 units; black line, mean; grey lines, mean ± s.e.). Values are means ± standard error.
Supplementary Figure 9 Ex vivo characterization of Jaws-expressing dentate granule cells in acute hippocampal slice.
(a) Epifluorescence image from Jaws-GFP expressing hippocampus, 4 weeks post-injection. Blue indicates DAPI staining, green indicates GFP fluorescence. Scale bar, 1 mm. (b) Physiological properties upon opsin expression at 4 weeks post-injection in dentate granule cells, including cell input resistance, resting potential, electrically evoked action potential threshold, electrically evoked action potential amplitude. (c) Physiological properties for Jaws-expressing and wildtype dentate granule cells upon red light illumination, including peak and steady-state photocurrent, peak and steady-state photopotential, and post-illumination rebound voltages. n = 12 for Jaws-positive cells, n = 15 for wildtype cells, throughout this panel. Illumination was 1 second at 68 mW/mm2, 625 nm. Values are means ± standard error. Statistics for panel b: * P < 0.05. Panel b was a Student's t-test.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 and Supplementary Table 1 (PDF 3461 kb)
Rights and permissions
About this article
Cite this article
Chuong, A., Miri, M., Busskamp, V. et al. Noninvasive optical inhibition with a red-shifted microbial rhodopsin. Nat Neurosci 17, 1123–1129 (2014). https://doi.org/10.1038/nn.3752
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3752
This article is cited by
-
The cerebellum directly modulates the substantia nigra dopaminergic activity
Nature Neuroscience (2024)
-
Thalamic nucleus reuniens coordinates prefrontal-hippocampal synchrony to suppress extinguished fear
Nature Communications (2023)
-
Cardiac optogenetics: shining light on signaling pathways
Pflügers Archiv - European Journal of Physiology (2023)
-
Genetic Approaches for Neural Circuits Dissection in Non-human Primates
Neuroscience Bulletin (2023)
-
A CRISPR toolbox for generating intersectional genetic mouse models for functional, molecular, and anatomical circuit mapping
BMC Biology (2022)