Abstract
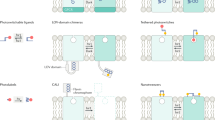
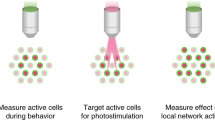
The optical neuroscience revolution is transforming how we study neural circuits. By providing a precise way to manipulate endogenous neuronal signaling proteins, it also has the potential to transform our understanding of molecular neuroscience. Recent advances in chemical biology have produced light-sensitive compounds that photoregulate a wide variety of proteins underlying signaling between and within neurons. Chemical tools for optopharmacology include caged agonists and antagonists and reversibly photoswitchable ligands. These reagents act on voltage-gated ion channels and neurotransmitter receptors, enabling control of neuronal signaling with a high degree of spatial and temporal precision. By covalently attaching photoswitch molecules to genetically tagged proteins, the newly emerging methodology of optogenetic pharmacology allows biochemically precise control in targeted subsets of neurons. Now that the tools for manipulating endogenous neuronal signaling proteins are available, they can be implemented in vivo to enhance our understanding of the molecular bases of brain function and dysfunctions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Fenno, L., Yizhar, O. & Deisseroth, K. The development and application of optogenetics. Annu. Rev. Neurosci. 34, 389–412 (2011).
Matsuzaki, M. et al. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat. Neurosci. 4, 1086–1092 (2001).
Ellis-Davies, G.C.R. Two-photon microscopy for chemical neuroscience. ACS Chem. Neurosci. 2, 185–197 (2011).
Fehrentz, T., Schönberger, M. & Trauner, D. Optochemical genetics. Angew. Chem. Int. Ed. Engl. 50, 12156–12182 (2011).
Shepherd, G.M.G., Pologruto, T.A. & Svoboda, K. Circuit analysis of experience-dependent plasticity in the developing rat barrel cortex. Neuron 38, 277–289 (2003).
Lutz, C. et al. Holographic photolysis of caged neurotransmitters. Nat. Methods 5, 821–827 (2008).
Canepari, M., Nelson, L.L., Papageorgiou, G.G., Corrie, J.E.T. & Ogden, D.D. Photochemical and pharmacological evaluation of 7-nitroindolinyl-and 4-methoxy-7-nitroindolinyl-amino acids as novel, fast caged neurotransmitters. J. Neurosci. Methods 112, 29–42 (2001).
Callaway, E.M. & Katz, L.C. Photostimulation using caged glutamate reveals functional circuitry in living brain slices. Proc. Natl. Acad. Sci. USA 90, 7661–7665 (1993).
Carter, A.G. & Sabatini, B.L. State-dependent calcium signaling in dendritic spines of striatal medium spiny neurons. Neuron 44, 483–493 (2004).
Donato, L. et al. Water-soluble, donor-acceptor biphenyl derivatives in the 2-(o-nitrophenyl)propyl series: highly efficient two-photon uncaging of the neurotransmitter γ-aminobutyric acid at λ = 800nm. Angew. Chem. Int. Ed. Engl. 51, 1840–1843 (2012).
Rial Verde, E.M., Zayat, L., Etchenique, R. & Yuste, R. Photorelease of GABA with visible light using an inorganic caging group. Front. Neural Circuits 2, 2 (2008).
Fino, E. et al. RuBi-glutamate: two-photon and visible-light photoactivation of neurons and dendritic spines. Front. Neural Circuits 3, 2 (2009).
Banghart, M.R. & Sabatini, B.L. Photoactivatable neuropeptides for spatiotemporally precise delivery of opioids in neural tissue. Neuron 73, 249–259 (2012).
Palma-Cerda, F. et al. New caged neurotransmitter analogs selective for glutamate receptor subtypes based on methoxynitroindoline and nitrophenylethoxycarbonyl caging groups. Neuropharmacology 63, 624–634 (2012).
Mourot, A. et al. Tuning photochromic ion channel blockers. ACS Chem. Neurosci. 2, 536–543 (2011).
Fortin, D.L. et al. Photochemical control of endogenous ion channels and cellular excitability. Nat. Methods 5, 331–338 (2008).
Banghart, M.R. et al. Photochromic blockers of voltage-gated potassium channels. Angew. Chem. Int. Ed. Engl. 48, 9097–9101 (2009).
Polosukhina, A. et al. Photochemical restoration of visual responses in blind mice. Neuron 75, 271–282 (2012).
Mourot, A. et al. Rapid optical control of nociception with an ion-channel photoswitch. Nat. Methods 9, 396–402 (2012).
Yue, L. et al. Robust photoregulation of GABAA receptors by allosteric modulation with a propofol analogue. Nature Commun. 3, 1095 (2012).
Volgraf, M. et al. Reversibly caged glutamate: a photochromic agonist of ionotropic glutamate receptors. J. Am. Chem. Soc. 129, 260–261 (2007).
Adesnik, H., Nicoll, R.A. & England, P.M. Photoinactivation of native AMPA receptors reveals their real-time trafficking. Neuron 48, 977–985 (2005).
Kokel, D. et al. Photochemical activation of TRPA1 channels in neurons and animals. Nat. Chem. Biol. 9, 257–263 (2013).
Banghart, M., Borges, K., Isacoff, E.Y., Trauner, D. & Kramer, R.H. Light-activated ion channels for remote control of neuronal firing. Nat. Neurosci. 7, 1381–1386 (2004).
Fortin, D.L. et al. Optogenetic photochemical control of designer K+ channels in mammalian neurons. J. Neurophysiol. 106, 488–496 (2011).
Sandoz, G., Levitz, J., Kramer, R.H. & Isacoff, E.Y. Optical control of endogenous proteins with a photoswitchable conditional subunit reveals a role for TREK1 in GABAB signaling. Neuron 74, 1005–1014 (2012).
Baranauskas, G., Tkatch, T., Nagata, K., Yeh, J.Z. & Surmeier, D.J. Kv3.4 subunits enhance the repolarizing efficiency of Kv3.1 channels in fast-spiking neurons. Nat. Neurosci. 6, 258–266 (2003).
Volgraf, M. et al. Allosteric control of an ionotropic glutamate receptor with an optical switch. Nat. Chem. Biol. 2, 47–52 (2006).
Szobota, S. et al. Remote control of neuronal activity with a light-gated glutamate receptor. Neuron 54, 535–545 (2007).
Wyart, C. et al. Optogenetic dissection of a behavioral module in the vertebrate spinal cord. Nature 461, 407–410 (2009).
Caporale, N. et al. LiGluR restores visual responses in rodent models of inherited blindness. Mol. Ther. 19, 1212–1219 (2011).
Howe, J.R. Homomeric and heteromeric ion channels formed from the kainate-type subunits GluR6 and KA2 have very small, but different, unitary conductances. J. Neurophysiol. 76, 510–519 (1996).
Feldbauer, K. et al. Channelrhodopsin-2 is a leaky proton pump. Proc. Natl. Acad. Sci. USA 106, 12317–12322 (2009).
Lester, H.A., Krouse, M.E.M., Nass, M.M.M., Wassermann, N.H. & Erlanger, B.F. A covalently bound photoisomerizable agonist: comparison with reversibly bound agonists at Electrophorus electroplaques. J. Gen. Physiol. 75, 207–232 (1980).
Tochitsky, I. et al. Optochemical control of genetically engineered neuronal nicotinic acetylcholine receptors. Nat. Chem. 4, 105–111 (2012).
Airan, R.D., Thompson, K.R., Fenno, L.E., Bernstein, H. & Deisseroth, K. Temporally precise in vivo control of intracellular signaling. Nature 458, 1025–1029 (2009).
Levitz, J. et al. Optical control of metabotropic glutamate receptors. Nat. Neurosci. (2013).
Schröder-Lang, S. et al. Fast manipulation of cellular cAMP level by light in vivo. Nat. Methods 4, 39–42 (2007).
Wu, Y.I. et al. A genetically encoded photoactivatable Rac controls the motility of living cells. Nature 461, 104–108 (2009).
Strickland, D. et al. TULIPs: tunable, light-controlled interacting protein tags for cell biology. Nat. Methods 9, 379–384 (2012).
Zhou, X.X., Chung, H.K., Lam, A.J. & Lin, M.Z. Optical control of protein activity by fluorescent protein domains. Science 338, 810–814 (2012).
Müller, K. et al. A red/far-red light-responsive bi-stable toggle switch to control gene expression in mammalian cells. Nucleic Acids Res. 41, e77 (2013).
Stuber, G.D., Hnasko, T.S., Britt, J.P., Edwards, R.H. & Bonci, A. Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum co-release glutamate. J. Neurosci. 30, 8229–8233 (2010).
Carter, M.E. et al. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat. Neurosci. 13, 1526–1533 (2010).
Donaldson, Z.R., Nautiyal, K.M., Ahmari, S.E. & Hen, R. Genetic approaches for understanding the role of serotonin receptors in mood and behavior. Curr. Opin. Neurobiol. 23, 399–406 (2013).
Bartos, M., Vida, I. & Jonas, P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat. Rev. Neurosci. 8, 45–56 (2007).
Adesnik, H., Bruns, W., Taniguchi, H., Huang, Z.J. & Scanziani, M. A neural circuit for spatial summation in visual cortex. Nature 490, 226–231 (2012).
Royer, S. et al. Control of timing, rate and bursts of hippocampal place cells by dendritic and somatic inhibition. Nat. Neurosci. 15, 769–775 (2012).
Noguchi, J. et al. In vivo two-photon uncaging of glutamate revealing the structure-function relationships of dendritic spines in the neocortex of adult mice. J. Physiol. (Lond.) 589, 2447–2457 (2011).
Stein, M. et al. Azo-propofols: photochromic potentiators of GABAA receptors. Angew. Chem. Int. Ed. Engl. 51, 10500–10504 (2012).
Acknowledgements
This work was supported by grants to R.H.K. from the National Eye Institute and the US National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Kramer, R., Mourot, A. & Adesnik, H. Optogenetic pharmacology for control of native neuronal signaling proteins. Nat Neurosci 16, 816–823 (2013). https://doi.org/10.1038/nn.3424
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3424
This article is cited by
-
The emergence of molecular systems neuroscience
Molecular Brain (2022)
-
Progress in DNA Aptamers as Recognition Components for Protein Functional Regulation
Chemical Research in Chinese Universities (2022)
-
Wireless multi-lateral optofluidic microsystems for real-time programmable optogenetics and photopharmacology
Nature Communications (2022)
-
Wireless and battery-free technologies for neuroengineering
Nature Biomedical Engineering (2021)
-
Light-controlled calcium signalling in prostate cancer and benign prostatic hyperplasia
Future Journal of Pharmaceutical Sciences (2020)