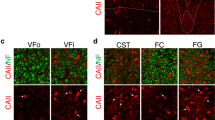
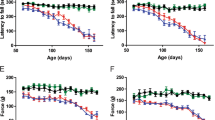
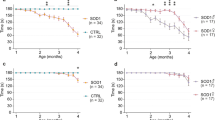
Abstract
Oligodendrocytes associate with axons to establish myelin and provide metabolic support to neurons. In the spinal cord of amyotrophic lateral sclerosis (ALS) mice, oligodendrocytes downregulate transporters that transfer glycolytic substrates to neurons and oligodendrocyte progenitors (NG2+ cells) exhibit enhanced proliferation and differentiation, although the cause of these changes in oligodendroglia is unknown. We found extensive degeneration of gray matter oligodendrocytes in the spinal cord of SOD1 (G93A) ALS mice prior to disease onset. Although new oligodendrocytes were formed, they failed to mature, resulting in progressive demyelination. Oligodendrocyte dysfunction was also prevalent in human ALS, as gray matter demyelination and reactive changes in NG2+ cells were observed in motor cortex and spinal cord of ALS patients. Selective removal of mutant SOD1 from oligodendroglia substantially delayed disease onset and prolonged survival in ALS mice, suggesting that ALS-linked genes enhance the vulnerability of motor neurons and accelerate disease by directly impairing the function of oligodendrocytes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Clement, A.M. et al. Wild-type nonneuronal cells extend survival of SOD1 mutant motor neurons in ALS mice. Science 302, 113–117 (2003).
Boillée, S. et al. Onset and progression in inherited ALS determined by motor neurons and microglia. Science 312, 1389–1392 (2006).
Yamanaka, K. et al. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat. Neurosci. 11, 251–253 (2008).
Ilieva, H., Polymenidou, M. & Cleveland, D.W. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J. Cell Biol. 187, 761–772 (2009).
Kang, S.H., Fukaya, M., Yang, J.K., Rothstein, J.D. & Bergles, D.E. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron 68, 668–681 (2010).
Magnus, T. et al. Adult glial precursor proliferation in mutant SOD1G93A mice. Glia 56, 200–208 (2008).
Nave, K.A. Myelination and support of axonal integrity by glia. Nature 468, 244–252 (2010).
Nave, K.A. & Trapp, B.D. Axon-glial signaling and the glial support of axon function. Annu. Rev. Neurosci. 31, 535–561 (2008).
Lee, Y. et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 487, 443–448 (2012).
Suzuki, A. et al. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 144, 810–823 (2011).
Rinholm, J.E. et al. Regulation of oligodendrocyte development and myelination by glucose and lactate. J. Neurosci. 31, 538–548 (2011).
Niebroj-Dobosz, I., Rafalowska, J., Fidzianska, A., Gadamski, R. & Grieb, P. Myelin composition of spinal cord in a model of amyotrophic lateral sclerosis (ALS) in SOD1G93A transgenic rats. Folia Neuropathol. 45, 236–241 (2007).
Cosottini, M. et al. Magnetization transfer imaging demonstrates a distributed pattern of microstructural changes of the cerebral cortex in amyotrophic lateral sclerosis. AJNR Am. J. Neuroradiol. 32, 704–708 (2011).
Rivers, L.E. et al. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat. Neurosci. 11, 1392–1401 (2008).
Zhu, X. et al. Age-dependent fate and lineage restriction of single NG2 cells. Development 138, 745–753 (2011).
Gurney, M.E. et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science 264, 1772–1775 (1994).
Dal Canto, M.C. & Gurney, M.E. Neuropathological changes in two lines of mice carrying a transgene for mutant human Cu,Zn SOD, and in mice overexpressing wild type human SOD: a model of familial amyotrophic lateral sclerosis (FALS). Brain Res. 676, 25–40 (1995).
Woodruff, R.H., Tekki-Kessaris, N., Stiles, C.D., Rowitch, D.H. & Richardson, W.D. Oligodendrocyte development in the spinal cord and telencephalon: common themes and new perspectives. Int. J. Dev. Neurosci. 19, 379–385 (2001).
Guo, F. et al. Pyramidal neurons are generated from oligodendroglial progenitor cells in adult piriform cortex. J. Neurosci. 30, 12036–12049 (2010).
Davalos, D. et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 8, 752–758 (2005).
Jamin, N., Junier, M.P., Grannec, G. & Cadusseau, J. Two temporal stages of oligodendroglial response to excitotoxic lesion in the gray matter of the adult rat brain. Exp. Neurol. 172, 17–28 (2001).
Wu, Y.J., Tang, Y.F., Xiao, Z.C., Bao, Z.M. & He, B.P. NG2 cells response to axonal alteration in the spinal cord white matter in mice with genetic disruption of neurofilament light subunit expression. Mol. Neurodegener. 3, 18 (2008).
Coutts, M., Kong, L.X. & Keirstead, H.S. A model of motor neuron loss: selective deficits after ricin injection. J. Neurotrauma 27, 1333–1342 (2010).
Henson, P.M., Bratton, D.L. & Fadok, V.A. Apoptotic cell removal. Curr. Biol. 11, R795–R805 (2001).
Locatelli, G. et al. Primary oligodendrocyte death does not elicit anti-CNS immunity. Nat. Neurosci. 15, 543–550 (2012).
Pohl, H.B. et al. Genetically induced adult oligodendrocyte cell death is associated with poor myelin clearance, reduced remyelination, and axonal damage. J. Neurosci. 31, 1069–1080 (2011).
Woodruff, R.H., Fruttiger, M., Richardson, W.D. & Franklin, R.J. Platelet-derived growth factor regulates oligodendrocyte progenitor numbers in adult CNS and their response following CNS demyelination. Mol. Cell Neurosci. 25, 252–262 (2004).
Chang, A., Nishiyama, A., Peterson, J., Prineas, J. & Trapp, B.D. NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J. Neurosci. 20, 6404–6412 (2000).
Pouly, S., Becher, B., Blain, M. & Antel, J.P. Expression of a homologue of rat NG2 on human microglia. Glia 27, 259–268 (1999).
Bu, J., Akhtar, N. & Nishiyama, A. Transient expression of the NG2 proteoglycan by a subpopulation of activated macrophages in an excitotoxic hippocampal lesion. Glia 34, 296–310 (2001).
Higuchi, M. et al. Axonal degeneration induced by targeted expression of mutant human tau in oligodendrocytes of transgenic mice that model glial tauopathies. J. Neurosci. 25, 9434–9443 (2005).
Yazawa, I. et al. Mouse model of multiple system atrophy alpha-synuclein expression in oligodendrocytes causes glial and neuronal degeneration. Neuron 45, 847–859 (2005).
Horner, P.J., Thallmair, M. & Gage, F.H. Defining the NG2-expressing cell of the adult CNS. J. Neurocytol. 31, 469–480 (2002).
Fünfschilling, U. et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 485, 517–521 (2012).
Bruijn, L.I. et al. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron 18, 327–338 (1997).
Pramatarova, A., Laganiere, J., Roussel, J., Brisebois, K. & Rouleau, G.A. Neuron-specific expression of mutant superoxide dismutase 1 in transgenic mice does not lead to motor impairment. J. Neurosci. 21, 3369–3374 (2001).
Rothstein, J.D., Van Kammen, M., Levey, A.I., Martin, L.J. & Kuncl, R.W. Selective loss of glial glutamate transporter GLT-1 in amyotrophic lateral sclerosis. Ann. Neurol. 38, 73–84 (1995).
Nagai, M. et al. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat. Neurosci. 10, 615–622 (2007).
Gong, Y.H., Parsadanian, A.S., Andreeva, A., Snider, W.D. & Elliott, J.L. Restricted expression of G86R Cu/Zn superoxide dismutase in astrocytes results in astrocytosis, but does not cause motoneuron degeneration. J. Neurosci. 20, 660–665 (2000).
Yamanaka, K. et al. Mutant SOD1 in cell types other than motor neurons and oligodendrocytes accelerates onset of disease in ALS mice. Proc. Natl. Acad. Sci. USA 105, 7594–7599 (2008).
Aebischer, J. et al. Elevated levels of IFNgamma and LIGHT in the spinal cord of patients with sporadic amyotrophic lateral sclerosis. Eur. J. Neurol. 19, 752–759 (2012).
Buntinx, M. et al. Cytokine-induced cell death in human oligodendroglial cell lines. I. Synergistic effects of IFN-gamma and TNF-alpha on apoptosis. J. Neurosci. Res. 76, 834–845 (2004).
Neumann, M. et al. TDP-43-positive white matter pathology in frontotemporal lobar degeneration with ubiquitin-positive inclusions. J. Neuropathol. Exp. Neurol. 66, 177–183 (2007).
Tu, P.H. et al. Glial cytoplasmic inclusions in white matter oligodendrocytes of multiple system atrophy brains contain insoluble alpha-synuclein. Ann. Neurol. 44, 415–422 (1998).
Komori, T. Tau-positive glial inclusions in progressive supranuclear palsy, corticobasal degeneration and Pick's disease. Brain Pathol. 9, 663–679 (1999).
Bauer, J. et al. Endoplasmic reticulum stress in PLP-overexpressing transgenic rats: gray matter oligodendrocytes are more vulnerable than white matter oligodendrocytes. J. Neuropathol. Exp. Neurol. 61, 12–22 (2002).
Chong, S.Y. et al. Neurite outgrowth inhibitor Nogo-A establishes spatial segregation and extent of oligodendrocyte myelination. Proc. Natl. Acad. Sci. USA 109, 1299–1304 (2012).
Dutta, R. & Trapp, B.D. Pathogenesis of axonal and neuronal damage in multiple sclerosis. Neurology 68, S22–S31 (2007).
Rudick, R.A. & Trapp, B.D. Gray-matter injury in multiple sclerosis. N. Engl. J. Med. 361, 1505–1506 (2009).
Doerflinger, N.H., Macklin, W.B. & Popko, B. Inducible site-specific recombination in myelinating cells. Genesis 35, 63–72 (2003).
Novak, A., Guo, C., Yang, W., Nagy, A. & Lobe, C.G. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis 28, 147–155 (2000).
Srinivas, S. et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 1, 4 (2001).
Muzumdar, M.D., Tasic, B., Miyamichi, K., Li, L. & Luo, L. A global double-fluorescent Cre reporter mouse. Genesis 45, 593–605 (2007).
Gong, S. et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature 425, 917–925 (2003).
Acknowledgements
We thank N. Ye, I. Srivastava, S. Singh and T. Le for their excellent technical support, M. Pucak for help with Imaris software operation and quantitative analysis, J. Carmen for her contributions to the Cre/lox animal study, and H. Zhang at the Johns Hopkins University School of Public Health FACS core for assistance with NG2+ cell isolation. We thank B. Trapp (Cleveland Clinic Lerner Research Institute) for advice regarding human NG2+ cell staining, R. Dutta (Cleveland Clinic Lerner Research Institute) for advice regarding protein extraction from human tissues and B. Popko (University of Chicago) for providing Plp1-creER mice. Human samples were provided by R. Bowser (Barrow Neurological Institute), K. Trevor (SACTL-VA Biorepository Trust), T. Hyde (Lieber Institute for Brain Development and the Johns Hopkins School of Medicine), J. Glass (Emory Alzheimer's Disease Research Center, 5P50AG025688-07), and the Johns Hopkins School of Medicine Department of Neuropathology. We thank E. Mosmiller for helping with patient demographic information. We also thank A. Agarwal and A. Langseth for critical discussions. This work was supported by grants from P2ALS (D.E.B. and J.D.R.), the US National Institutes of Health (NS27036 to D.W.C., NS33958 to J.D.R. and NS051509 to D.E.B.), the ALS Association (4ZMUDE to Y.L.), the Robert Packard Center for ALS Research at Johns Hopkins, and the Brain Science Institute.
Author information
Authors and Affiliations
Contributions
S.H.K., Y.L., J.D.R. and D.E.B. designed the experiments. S.H.K. carried out the experiments involving NG2+ cell proliferation, cell fate analysis of NG2+ cells and oligodendrocytes, and immunohistochemical analysis of oligodendrocyte structure (Figs. 1,2,3,4,5,6 and Supplementary Figs. 1–3 and 5). Y.L. performed western blot analysis from SOD1 (G93A) mice (Supplementary Fig. 7) and ALS patients (Supplementary Fig. 8), histological analysis of human tissue (Fig. 7), analysis of ricin-injected mice (Supplementary Fig. 4), and analysis of the SOD1 (G37R)–deleted mice (Fig. 8 and Supplementary Fig. 9). M.F. performed the electron microscopic and immunogold analysis (Fig. 6 and Supplementary Fig. 6). I.L. assisted with the ricin injections. D.W.C. provided the loxSOD1 (G37R) mice. L.W.O. provided assistance with analysis of the human ALS samples. S.H.K., Y.L., J.D.R. and D.E.B. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–11 and Supplementary Table 1 (PDF 3864 kb)
Rights and permissions
About this article
Cite this article
Kang, S., Li, Y., Fukaya, M. et al. Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis. Nat Neurosci 16, 571–579 (2013). https://doi.org/10.1038/nn.3357
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3357
This article is cited by
-
Disruption of MAM integrity in mutant FUS oligodendroglial progenitors from hiPSCs
Acta Neuropathologica (2024)
-
Axonal energy metabolism, and the effects in aging and neurodegenerative diseases
Molecular Neurodegeneration (2023)
-
Regulation of cortical hyperexcitability in amyotrophic lateral sclerosis: focusing on glial mechanisms
Molecular Neurodegeneration (2023)
-
Elevated peripheral levels of receptor-interacting protein kinase 1 (RIPK1) and IL-8 as biomarkers of human amyotrophic lateral sclerosis
Signal Transduction and Targeted Therapy (2023)
-
Integrative transcriptomic analysis of the amyotrophic lateral sclerosis spinal cord implicates glial activation and suggests new risk genes
Nature Neuroscience (2023)