Abstract
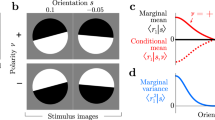
The activity of cortical neurons in sensory areas covaries with perceptual decisions, a relationship that is often quantified by choice probabilities. Although choice probabilities have been measured extensively, their interpretation has remained fraught with difficulty. We derive the mathematical relationship between choice probabilities, read-out weights and correlated variability in the standard neural decision-making model. Our solution allowed us to prove and generalize earlier observations on the basis of numerical simulations and to derive new predictions. Notably, our results indicate how the read-out weight profile, or decoding strategy, can be inferred from experimentally measurable quantities. Furthermore, we developed a test to decide whether the decoding weights of individual neurons are optimal for the task, even without knowing the underlying correlations. We confirmed the practicality of our approach using simulated data from a realistic population model. Thus, our findings provide a theoretical foundation for a growing body of experimental results on choice probabilities and correlations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Parker, A.J. & Newsome, W. Sense and the single neuron: probing the physiology of perception. Annu. Rev. Neurosci. 21, 227–277 (1998).
Grunewald, A., Bradley, D. & Andersen, R. Neural correlates of structure-from-motion perception in macaque V1 and MT. J. Neurosci. 22, 6195–6207 (2002).
Nienborg, H. & Cumming, B. Macaque V2 neurons, but not V1 neurons, show choice-related activity. J. Neurosci. 26, 9567–9578 (2006).
Uka, T., Tanabe, S., Watanabe, M. & Fujita, I. Neural correlates of fine depth discrimination in monkey inferior temporal cortex. J. Neurosci. 25, 10796–10802 (2005).
Britten, K.H., Newsome, W., Shadlen, M., Celebrini, S. & Movshon, J. A relationship between behavioral choice and the visual responses of neurons in macaque MT. Vis. Neurosci. 13, 87–100 (1996).
Dodd, J.V., Krug, K., Cumming, B. & Parker, A. Perceptually bistable three-dimensional figures evoke high choice probabilities in cortical area MT. J. Neurosci. 21, 4809–4821 (2001).
Cook, E.P. & Maunsell, J. Dynamics of neuronal responses in macaque MT and VIP during motion detection. Nat. Neurosci. 5, 985–994 (2002).
Parker, A.J., Krug, K. & Cumming, B. Neuronal activity and its links with the perception of multi-stable figures. Phil. Trans. R. Soc. Lond. B 357, 1053–1062 (2002).
Uka, T. & DeAngelis, G. Contribution of area MT to stereoscopic depth perception: choice-related response modulations reflect task strategy. Neuron 42, 297–310 (2004).
Liu, J. & Newsome, W. Correlation between speed perception and neural activity in the middle temporal visual area. J. Neurosci. 25, 711–722 (2005).
Purushothaman, G. & Bradley, D. Neural population code for fine perceptual decisions in area MT. Nat. Neurosci. 8, 99–106 (2005).
Law, C.T. & Gold, J. Neural correlates of perceptual learning in a sensory-motor, but not a sensory, cortical area. Nat. Neurosci. 11, 505–513 (2008).
Celebrini, S. & Newsome, W. Neuronal and psychophysical sensitivity to motion signals in extrastriate area MST of the macaque monkey. J. Neurosci. 14, 4109–4124 (1994).
Shadlen, M.N., Britten, K., Newsome, W. & Movshon, J. A computational analysis of the relationship between neuronal and behavioral responses to visual motion. J. Neurosci. 16, 1486–1510 (1996).
Cohen, M.R. & Newsome, W. Estimates of the contribution of single neurons to perception depend on timescale and noise correlation. J. Neurosci. 29, 6635–6648 (2009).
Nienborg, H. & Cumming, B. Correlations between the activity of sensory neurons and behavior: how much do they tell us about a neuron's causality? Curr. Opin. Neurobiol. 20, 376–381 (2010).
Gold, J.I. & Shadlen, M. The neural basis of decision making. Annu. Rev. Neurosci. 30, 535–574 (2007).
Newsome, W.T., Britten, K. & Movshon, J. Neuronal correlates of a perceptual decision. Nature 341, 52–54 (1989).
Nienborg, H. & Cumming, B. Decision-related activity in sensory neurons reflects more than a neuron's causal effect. Nature 459, 89–92 (2009).
Nienborg, H., Cohen, M. & Cumming, B.G. Decision-related activity in sensory neurons: correlations among neurons and with behavior. Annu. Rev. Neurosci. 35, 463–483 (2012).
Wang, X.J. Decision making in recurrent neuronal circuits. Neuron 60, 215–234 (2008).
Gu, Y., Angelaki, D. & DeAngelis, G. Neural correlates of multisensory cue integration in macaque MSTd. Nat. Neurosci. 11, 1201–1210 (2008).
Nienborg, H. & Cumming, B. Psychophysically measured task strategy for disparity discrimination is reflected in V2 neurons. Nat. Neurosci. 10, 1608–1614 (2007).
Abbott, L.F. & Dayan, P. The effect of correlated variability on the accuracy of a population code. Neural Comput. 11, 91–101 (1999).
Chen, Y., Geisler, W. & Seidemann, E. Optimal decoding of correlated neural population responses in the primate visual cortex. Nat. Neurosci. 9, 1412–1420 (2006).
Cohen, M.R. & Newsome, W. Context-dependent changes in functional circuitry in visual area MT. Neuron 60, 162–173 (2008).
Law, C.T. & Gold, J. Reinforcement learning can account for associative and perceptual learning on a visual-decision task. Nat. Neurosci. 12, 655–663 (2009).
Bishop, C. Pattern Recognition and Machine Learning (Springer, New York, 2006).
Averbeck, B.B., Latham, P. & Pouget, A. Neural correlations, population coding and computation. Nat. Rev. Neurosci. 7, 358–366 (2006).
Cohen, M.R. & Kohn, A. Measuring and interpreting neuronal correlations. Nat. Neurosci. 14, 811–819 (2011).
Gu, Y. et al. Perceptual learning reduces interneuronal correlations in macaque visual cortex. Neuron 71, 750–761 (2011).
Buzsáki, G. Large-scale recording of neuronal ensembles. Nat. Neurosci. 7, 446–451 (2004).
Kerr, J.N. & Denk, W. Imaging in vivo: watching the brain in action. Nat. Rev. Neurosci. 9, 195–205 (2008).
Stevenson, I.H. & Kording, K. How advances in neural recording affect data analysis. Nat. Neurosci. 14, 139–142 (2011).
Shadlen, M.N. & Newsome, W. The variable discharge of cortical neurons: implications for connectivity, computation and information coding. J. Neurosci. 18, 3870–3896 (1998).
Liu, J. & Newsome, W. T. Local field potential in cortical area MT: stimulus tuning and behavioral correlations. J. Neurosci. 26, 7779–7790 (2006).
Marder, E. Variability, compensation, and modulation in neurons and circuits. Proc. Natl. Acad. Sci. USA 108 (suppl. 3): 15542–15548 (2011).
Padmanabhan, K. & Urban, N. Intrinsic biophysical diversity decorrelates neuronal firing while increasing information content. Nat. Neurosci. 13, 1276–1282 (2010).
Ecker, A.S., Berens, P., Tolias, A. & Bethge, M. The effect of noise correlations in populations of diversely tuned neurons. J. Neurosci. 31, 14272–14283 (2011).
Churchland, M.M. & Shenoy, K. Temporal complexity and heterogeneity of single-neuron activity in premotor and motor cortex. J. Neurophysiol. 97, 4235–4257 (2007).
Jazayeri, M. Probabilistic sensory recoding. Curr. Opin. Neurobiol. 18, 431–437 (2008).
Acknowledgements
We thank A. Ecker and P. Berens for stimulating discussions and detailed comments on an earlier version of the manuscript, and H. Nienborg and B.G. Cumming for many helpful conversations. This work was partially supported by the German Ministry of Education, Science, Research and Technology through the Bernstein Award (FKZ 01GQ0601) (M.B.), the Bernstein Center for Computational Neuroscience (FKZ 01GQ1002), the German Excellency Initiative through the Centre for Integrative Neuroscience Tübingen (EXC307) and the European Commission (FP7-ICT-257005). R.M.H. acknowledges the hospitality of the Fiser laboratory at Brandeis University where this study was completed and financial support from the Swartz Foundation. Part of this research was done while J.H.M. was at the Gatsby Computational Neuroscience Unit, University College London.
Author information
Authors and Affiliations
Contributions
R.M.H. conceived the research. R.M.H. and S.G. performed the analytical calculations and R.M.H. performed the simulations. All of the authors discussed the results. R.M.H. wrote the paper with contributions from the other authors. J.H.M. and M.B. advised at all stages.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Note, Supplementary Figures 1–11 (PDF 3416 kb)
Rights and permissions
About this article
Cite this article
Haefner, R., Gerwinn, S., Macke, J. et al. Inferring decoding strategies from choice probabilities in the presence of correlated variability. Nat Neurosci 16, 235–242 (2013). https://doi.org/10.1038/nn.3309
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3309
This article is cited by
-
Behavioral read-out from population value signals in primate orbitofrontal cortex
Nature Neuroscience (2023)
-
Individual risk attitudes arise from noise in neurocognitive magnitude representations
Nature Human Behaviour (2023)
-
Distributed context-dependent choice information in mouse posterior cortex
Nature Communications (2023)
-
A neural correlate of perceptual segmentation in macaque middle temporal cortical area
Nature Communications (2022)
-
Revealing nonlinear neural decoding by analyzing choices
Nature Communications (2021)