Abstract
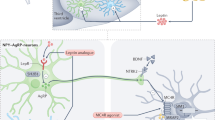
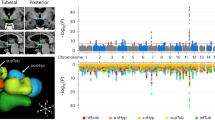
Over the past 20 years, genetic studies have illuminated critical pathways in the hypothalamus and brainstem mediating energy homeostasis, such as the melanocortin, leptin, 5-hydroxytryptamine and brain-derived neurotrophic factor signaling axes. The identification of these pathways necessary for appropriate appetitive responses to energy state has yielded insight into normal homeostatic processes. Although monogenic alterations in each of these axes result in severe obesity, such cases remain rare. The major burden of disease is carried by those with common obesity, which has so far resisted yielding meaningful biological insights. Recent progress into the etiology of common obesity has been made with genome-wide association studies. Such studies now reveal more than 32 different candidate obesity genes, most of which are highly expressed or known to act in the CNS, emphasizing, as in rare monogenic forms of obesity, the role of the brain in predisposition to obesity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Stunkard, A.J., Foch, T.T. & Hrubec, Z. A twin study of human obesity. J. Am. Med. Assoc. 256, 51–54 (1986).
Stunkard, A.J., Harris, J.R., Pedersen, N.L. & McClearn, G.E. The body-mass index of twins who have been reared apart. N. Engl. J. Med. 322, 1483–1487 (1990).
Cone, R.D. Anatomy and regulation of the central melanocortin system. Nat. Neurosci. 8, 571–578 (2005).
Bertagna, X. Proopiomelanocortin-derived peptides. Endocrinol. Metab. Clin. North Am. 23, 467–485 (1994).
Castro, M.G. & Morrison, E. Post-translational processing of proopiomelanocortin in the pituitary and in the brain. Crit. Rev. Neurobiol. 11, 35–57 (1997).
Ollmann, M.M. et al. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science 278, 135–138 (1997).
Fan, W., Boston, B.A., Kesterson, R.A., Hruby, V.J. & Cone, R.D. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature 385, 165–168 (1997).
Cowley, M.A. et al. Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron 24, 155–163 (1999).
Aponte, Y., Atasoy, D. & Sternson, S.M. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat. Neurosci. 14, 351–355 (2011).
Krashes, M.J. et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J. Clin. Invest. 121, 1424–1428 (2011).
Atasoy, D., Betley, J.N., Su, H.H. & Sternson, S.M. Deconstruction of a neural circuit for hunger. Nature 488, 172–177 (2012).
Erickson, J.C., Clegg, K.E. & Palmiter, R.D. Sensitivity to leptin and susceptibility to seizures of mice lacking neuropeptide Y. Nature 381, 415–418 (1996).
Erickson, J.C., Hollopeter, G. & Palmiter, R.D. Attenuation of the obesity syndrome of ob/ob mice by the loss of neuropeptide Y. Science 274, 1704–1707 (1996).
Qian, S. et al. Neither agouti-related protein nor neuropeptide Y is critically required for the regulation of energy homeostasis in mice. Mol. Cell. Biol. 22, 5027–5035 (2002).
Tong, Q., Ye, C.P., Jones, J.E., Elmquist, J.K. & Lowell, B.B. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat. Neurosci. 11, 998–1000 (2008).
Wortley, K.E. et al. Agouti-related protein-deficient mice display an age-related lean phenotype. Cell Metab. 2, 421–427 (2005).
Bewick, G.A. et al. Post-embryonic ablation of AgRP neurons in mice leads to a lean, hypophagic phenotype. FASEB J. 19, 1680–1682 (2005).
Gropp, E. et al. Agouti-related peptide-expressing neurons are mandatory for feeding. Nat. Neurosci. 8, 1289–1291 (2005).
Luquet, S., Perez, F.A., Hnasko, T.S. & Palmiter, R.D. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science 310, 683–685 (2005).
Xu, A.W. et al. Effects of hypothalamic neurodegeneration on energy balance. PLoS Biol. 3, e415 (2005).
Wu, Q., Boyle, M.P. & Palmiter, R.D. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell 137, 1225–1234 (2009).
Wu, Q., Clark, M.S. & Palmiter, R.D. Deciphering a neuronal circuit that mediates appetite. Nature 483, 594–597 (2012).
Challis, B.G. et al. Mice lacking pro-opiomelanocortin are sensitive to high-fat feeding but respond normally to the acute anorectic effects of peptide-YY3–36 . Proc. Natl. Acad. Sci. USA 101, 4695–4700 (2004).
Huszar, D. et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 88, 131–141 (1997).
Yaswen, L., Diehl, N., Brennan, M.B. & Hochgeschwender, U. Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nat. Med. 5, 1066–1070 (1999).
Krude, H. et al. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat. Genet. 19, 155–157 (1998).
Vaisse, C., Clement, K., Guy-Grand, B. & Froguel, P. A frameshift mutation in human MC4R is associated with a dominant form of obesity. Nat. Genet. 20, 113–114 (1998).
Yeo, G.S. et al. A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nat. Genet. 20, 111–112 (1998).
Farooqi, I.S. et al. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N. Engl. J. Med. 348, 1085–1095 (2003).
Kishi, T. et al. Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J. Comp. Neurol. 457, 213–235 (2003).
Balthasar, N. et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 123, 493–505 (2005).
Rossi, J. et al. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab. 13, 195–204 (2011).
Friedman, J.M. & Halaas, J.L. Leptin and the regulation of body weight in mammals. Nature 395, 763–770 (1998).
Zhang, Y. et al. Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425–432 (1994).
Chen, H. et al. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell 84, 491–495 (1996).
Montague, C.T. et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature 387, 903–908 (1997).
Farooqi, I.S. et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N. Engl. J. Med. 341, 879–884 (1999).
Pelleymounter, M.A. et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science 269, 540–543 (1995).
Heymsfield, S.B. et al. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. J. Am. Med. Assoc. 282, 1568–1575 (1999).
Ahima, R.S. et al. Role of leptin in the neuroendocrine response to fasting. Nature 382, 250–252 (1996).
de Luca, C. et al. Complete rescue of obesity, diabetes, and infertility in db/db mice by neuron-specific LEPR-B transgenes. J. Clin. Invest. 115, 3484–3493 (2005).
Cowley, M.A. et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411, 480–484 (2001).
Balthasar, N. et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron 42, 983–991 (2004).
van de Wall, E. et al. Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology 149, 1773–1785 (2008).
Dhillon, H. et al. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron 49, 191–203 (2006).
Leinninger, G.M. et al. Leptin action via neurotensin neurons controls orexin, the mesolimbic dopamine system and energy balance. Cell Metab. 14, 313–323 (2011).
Hayes, M.R. et al. Endogenous leptin signaling in the caudal nucleus tractus solitarius and area postrema is required for energy balance regulation. Cell Metab. 11, 77–83 (2010).
Hommel, J.D. et al. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron 51, 801–810 (2006).
Yadav, V.K. et al. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell 138, 976–989 (2009).
Lam, D.D. et al. Leptin does not directly affect CNS serotonin neurons to influence appetite. Cell Metab. 13, 584–591 (2011).
Lam, D.D. & Heisler, L.K. Serotonin and energy balance: molecular mechanisms and implications for type 2 diabetes. Expert Rev. Mol. Med. 9, 1–24 (2007).
Garfield, A.S. & Heisler, L.K. Pharmacological targeting of the serotonergic system for the treatment of obesity. J. Physiol. (Lond.) 587, 49–60 (2009).
Heisler, L.K. et al. Activation of central melanocortin pathways by fenfluramine. Science 297, 609–611 (2002).
Heisler, L.K. et al. Serotonin reciprocally regulates melanocortin neurons to modulate food intake. Neuron 51, 239–249 (2006).
Fidler, M.C. et al. A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: the BLOSSOM trial. J. Clin. Endocrinol. Metab. 96, 3067–3077 (2011).
Sohn, J.W. et al. Serotonin 2C receptor activates a distinct population of arcuate pro-opiomelanocortin neurons via TRPC channels. Neuron 71, 488–497 (2011).
Xu, Y. et al. 5–HT2CRs expressed by pro-opiomelanocortin neurons regulate energy homeostasis. Neuron 60, 582–589 (2008).
Xu, Y. et al. A serotonin and melanocortin circuit mediates d-fenfluramine anorexia. J. Neurosci. 30, 14630–14634 (2010).
Barde, Y.A. The nerve growth factor family. Prog. Growth Factor Res. 2, 237–248 (1990).
Snider, W.D. Functions of the neurotrophins during nervous system development: what the knockouts are teaching us. Cell 77, 627–638 (1994).
Kernie, S.G., Liebl, D.J. & Parada, L.F. BDNF regulates eating behavior and locomotor activity in mice. EMBO J. 19, 1290–1300 (2000).
Rios, M. et al. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol. Endocrinol. 15, 1748–1757 (2001).
Xu, B. et al. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat. Neurosci. 6, 736–742 (2003).
Gray, J. et al. Hyperphagia, severe obesity, impaired cognitive function, and hyperactivity associated with functional loss of one copy of the brain-derived neurotrophic factor (BDNF) gene. Diabetes 55, 3366–3371 (2006).
Yeo, G.S. et al. A de novo mutation affecting human TrkB associated with severe obesity and developmental delay. Nat. Neurosci. 7, 1187–1189 (2004).
Pelleymounter, M.A., Cullen, M.J. & Wellman, C.L. Characteristics of BDNF-induced weight loss. Exp. Neurol. 131, 229–238 (1995).
Bariohay, B. et al. Brain-derived neurotrophic factor/tropomyosin-related kinase receptor type B signaling is a downstream effector of the brainstem melanocortin system in food intake control. Endocrinology 150, 2646–2653 (2009).
Spaeth, A.M., Kanoski, S.E., Hayes, M.R. & Grill, H.J. TrkB receptor signaling in the nucleus tractus solitarius mediates the food intake suppressive effects of hindbrain BDNF and leptin. Am. J. Physiol. Endocrinol. Metab. 302, E1252–E1260 (2012).
Nicholson, J.R., Peter, J.C., Lecourt, A.C., Barde, Y.A. & Hofbauer, K.G. Melanocortin-4 receptor activation stimulates hypothalamic brain-derived neurotrophic factor release to regulate food intake, body temperature and cardiovascular function. J. Neuroendocrinol. 19, 974–982 (2007).
Komori, T., Morikawa, Y., Nanjo, K. & Senba, E. Induction of brain-derived neurotrophic factor by leptin in the ventromedial hypothalamus. Neuroscience 139, 1107–1115 (2006).
Godar, R. et al. Reduction of high-fat diet-induced obesity after chronic administration of brain-derived neurotrophic factor in the hypothalamic ventromedial nucleus. Neuroscience 194, 36–52 (2011).
Unger, T.J., Calderon, G.A., Bradley, L.C., Sena-Esteves, M. & Rios, M. Selective deletion of Bdnf in the ventromedial and dorsomedial hypothalamus of adult mice results in hyperphagic behavior and obesity. J. Neurosci. 27, 14265–14274 (2007).
Tran, P.V. et al. Diminished hypothalamic bdnf expression and impaired VMH function are associated with reduced SF-1 gene dosage. J. Comp. Neurol. 498, 637–648 (2006).
Frayling, T.M. et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 316, 889–894 (2007).
Tung, Y.C. & Yeo, G.S. From GWAS to biology: lessons from FTO. Ann. NY Acad. Sci. 1220, 162–171 (2011).
Boissel, S. et al. Loss-of-function mutation in the dioxygenase-encoding FTO gene causes severe growth retardation and multiple malformations. Am. J. Hum. Genet. 85, 106–111 (2009).
Fischer, J. et al. Inactivation of the Fto gene protects from obesity. Nature 458, 894–898 (2009).
Gerken, T. et al. The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science 318, 1469–1472 (2007).
Tung, Y.C. et al. Hypothalamic-specific manipulation of Fto, the ortholog of the human obesity gene FTO, affects food intake in rats. PLoS ONE 5, e8771 (2010).
Speliotes, E.K. et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat. Genet. 42, 937–948 (2010).
Willer, C.J. et al. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat. Genet. 41, 25–34 (2009).
Loos, R.J. et al. Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nat. Genet. 40, 768–775 (2008).
Ren, D. et al. Neuronal SH2B1 is essential for controlling energy and glucose homeostasis. J. Clin. Invest. 117, 397–406 (2007).
Funatsu, N. et al. Characterization of a novel rat brain glycosylphosphatidylinositol-anchored protein (Kilon), a member of the IgLON cell adhesion molecule family. J. Biol. Chem. 274, 8224–8230 (1999).
Jurvansuu, J.M. & Goldman, A. Obesity risk gene TMEM18 encodes a sequence-specific DNA-binding protein. PLoS ONE 6, e25317 (2011).
Morris, H.V. et al. Alpha2-containing GABAA receptors are involved in mediating stimulant effects of cocaine. Pharmacol. Biochem. Behav. 90, 9–18 (2008).
Suviolahti, E. et al. The SLC6A14 gene shows evidence of association with obesity. J. Clin. Invest. 112, 1762–1772 (2003).
Vimaleswaran, K.S. et al. Association between serotonin 5-HT-2C receptor gene (HTR2C) polymorphisms and obesity- and mental health-related phenotypes in a large population-based cohort. Int. J. Obes. (Lond.) 34, 1028–1033 (2010).
Acknowledgements
G.S.H.Y. is supported by the UK Medical Research Council Centre for Obesity and Related Metabolic Disorders (MRC-CORD) and the European Union (FP7-HEALTH-2009-241592 EurOCHIP and FP7-HEALTH-266408 Full4Health). L.K.H. is supported by the Wellcome Trust (WT081713; WT090812).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Yeo, G., Heisler, L. Unraveling the brain regulation of appetite: lessons from genetics. Nat Neurosci 15, 1343–1349 (2012). https://doi.org/10.1038/nn.3211
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3211
This article is cited by
-
Glial cells as integrators of peripheral and central signals in the regulation of energy homeostasis
Nature Metabolism (2022)
-
Neural circuit control of innate behaviors
Science China Life Sciences (2022)
-
Effects of Heterozygous Variants in the Leptin-Melanocortin Pathway on Roux-en-Y Gastric Bypass Outcomes: a 15-Year Case–Control Study
Obesity Surgery (2022)
-
Activation of the Melanocortin-4 receptor signaling by α-MSH stimulates nerve-dependent mouse digit regeneration
Cell Regeneration (2021)
-
Brain JNK and metabolic disease
Diabetologia (2021)