Abstract
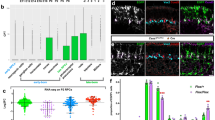
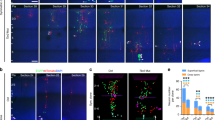
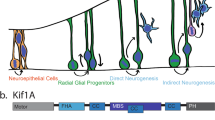
Neurogenesis in the developing neocortex relies on the ability of radial glial progenitor cells (RGCs) to switch from proliferative to differentiative neuron-generating divisions, but the molecular mechanisms that control this switch in a correct temporal manner are not well understood. Here, we show that DOCK7, a member of the DOCK180 family of proteins, regulates RGC proliferation versus differentiation. Silencing of DOCK7 in RGCs of developing mouse embryos impedes neuronal differentiation and maintains cells as cycling progenitors. In contrast, DOCK7 overexpression promotes RGC differentiation to basal progenitors and neurons. We further present evidence that DOCK7 influences neurogenesis by controlling apically directed interkinetic nuclear migration of RGCs. DOCK7 exerts its effects by antagonizing the microtubule growth-promoting function of the centrosome-associated protein TACC3. Thus, DOCK7 interaction with TACC3 controls interkinetic nuclear migration and the genesis of neurons from RGCs during cortical development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
McConnell, S.K. Constructing the cerebral cortex: neurogenesis and fate determination. Neuron 15, 761–768 (1995).
Götz, M. & Huttner, W.B. The cell biology of neurogenesis. Nat. Rev. Mol. Cell Biol. 6, 777–788 (2005).
Farkas, L.M. & Huttner, W.B. The cell biology of neural stem and progenitor cells and its significance for their proliferation versus differentiation during mammalian brain development. Curr. Opin. Cell Biol. 20, 707–715 (2008).
Miyata, T., Kawaguchi, D., Kawaguchi, A. & Gotoh, Y. Mechanisms that regulate the number of neurons during mouse neocortical development. Curr. Opin. Neurobiol. 20, 22–28 (2010).
Johansson, P.A., Cappello, S. & Götz, M. Stem cells niches during development–lessons from the cerebral cortex. Curr. Opin. Neurobiol. 20, 400–407 (2010).
Miyata, T., Kawaguchi, A., Okano, H. & Ogawa, M. Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron 31, 727–741 (2001).
Tamamaki, N., Nakamura, K., Okamoto, K. & Kaneko, T. Radial glia is a progenitor of neocortical neurons in the developing cerebral cortex. Neurosci. Res. 41, 51–60 (2001).
Noctor, S.C., Flint, A.C., Weissman, T.A., Dammerman, R.S. & Kriegstein, A.R. Neurons derived from radial glial cells establish radial units in neocortex. Nature 409, 714–720 (2001).
Noctor, S.C., Martinez-Cerdeno, V., Ivic, L. & Kriegstein, A.R. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat. Neurosci. 7, 136–144 (2004).
Anthony, T.E., Klein, C., Fishell, G. & Heintz, N. Radial glia serve as neuronal progenitors in all regions of the central nervous system. Neuron 41, 881–890 (2004).
Malatesta, P., Hartfuss, E. & Götz, M. Isolation of radial glial cells by fluorescent-activated cell sorting reveals a neuronal lineage. Development 127, 5253–5263 (2000).
Latasa, M.J., Cisneros, E. & Frade, J.M. Cell cycle control of Notch signaling and the functional regionalization of the neuroepithelium during vertebrate neurogenesis. Int. J. Dev. Biol. 53, 895–908 (2009).
Taverna, E. & Huttner, W.B. Neural progenitor nuclei IN motion. Neuron 67, 906–914 (2010).
Willardsen, M.I. & Link, B.A. Cell biological regulation of division fate in vertebrate neuroepithelial cells. Dev. Dyn. 240, 1865–1879 (2011).
Takahashi, T., Nowakowski, R.S. & Caviness, V.S. Jr. The leaving or Q fraction of the murine cerebral proliferative epithelium: a general model of neocortical neuronogenesis. J. Neurosci. 16, 6183–6196 (1996).
Noctor, S.C., Martinez-Cerdeno, V. & Kriegstein, A.R. Distinct behaviors of neural stem and progenitor cells underlie cortical neurogenesis. J. Comp. Neurol. 508, 28–44 (2008).
Haubensak, W., Attardo, A., Denk, W. & Huttner, W.B. Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: a major site of neurogenesis. Proc. Natl. Acad. Sci. USA 101, 3196–3201 (2004).
Miyata, T. et al. Asymmetric production of surface-dividing and non-surface-dividing cortical progenitor cells. Development 131, 3133–3145 (2004).
Doe, C.Q. Neural stem cells: balancing self-renewal with differentiation. Development 135, 1575–1587 (2008).
Manzini, M.C. & Walsh, C.A. What disorders of cortical development tell us about the cortex: one plus one does not always make two. Curr. Opin. Genet. Dev. 21, 333–339 (2011).
Bärenz, F., Mayilo, D. & Gruss, O.J. Centriolar satellites: Busy orbits around the centrosome. Eur. J. Cell Biol. 90, 983–989 (2011).
Dehay, C. & Kennedy, H. Cell-cycle control and cortical development. Nat. Rev. Neurosci. 8, 438–450 (2007).
Lange, C., Huttner, W.B. & Calegari, F. Cdk4/cyclinD1 overexpression in neural stem cells shortens G1, delays neurogenesis, and promotes the generation and expansion of basal progenitors. Cell Stem Cell 5, 320–331 (2009).
Bultje, R.S. et al. Mammalian Par3 regulates progenitor cell asymmetric division via notch signaling in the developing neocortex. Neuron 63, 189–202 (2009).
Wang, X. et al. Asymmetric centrosome inheritance maintains neural progenitors in the neocortex. Nature 461, 947–955 (2009).
Schwamborn, J.C., Berezikov, E. & Knoblich, J.A. The TRIM-NHL protein TRIM32 activates microRNAs and prevents self-renewal in mouse neural progenitors. Cell 136, 913–925 (2009).
Gauthier-Fisher, A. et al. Lfc and Tctex-1 regulate the genesis of neurons from cortical precursor cells. Nat. Neurosci. 12, 735–744 (2009).
Baye, L.M. & Link, B.A. Interkinetic nuclear migration and the selection of neurogenic cell divisions during vertebrate retinogenesis. J. Neurosci. 27, 10143–10152 (2007).
Del Bene, F., Wehman, A.M., Link, B.A. & Baier, H. Regulation of neurogenesis by interkinetic nuclear migration through an apical-basal notch gradient. Cell 134, 1055–1065 (2008).
Murciano, A., Zamora, J., Lopez-Sanchez, J. & Frade, J.M. Interkinetic nuclear movement may provide spatial clues to the regulation of neurogenesis. Mol. Cell. Neurosci. 21, 285–300 (2002).
Ge, X., Frank, C.L., Calderon de Anda, F. & Tsai, L.H. Hook3 interacts with PCM1 to regulate pericentriolar material assembly and the timing of neurogenesis. Neuron 65, 191–203 (2010).
Xie, Z. et al. Cep120 and TACCs control interkinetic nuclear migration and the neural progenitor pool. Neuron 56, 79–93 (2007).
Schenk, J., Wilsch-Brauninger, M., Calegari, F. & Huttner, W.B. Myosin II is required for interkinetic nuclear migration of neural progenitors. Proc. Natl. Acad. Sci. USA 106, 16487–16492 (2009).
Cappello, S. et al. The Rho-GTPase cdc42 regulates neural progenitor fate at the apical surface. Nat. Neurosci. 9, 1099–1107 (2006).
Tsai, J.W., Chen, Y., Kriegstein, A.R. & Vallee, R.B. LIS1 RNA interference blocks neural stem cell division, morphogenesis, and motility at multiple stages. J. Cell Biol. 170, 935–945 (2005).
Kosodo, Y. et al. Regulation of interkinetic nuclear migration by cell cycle-coupled active and passive mechanisms in the developing brain. EMBO J. 30, 1690–1704 (2011).
Tsai, J.W., Lian, W.N., Kemal, S., Kriegstein, A.R. & Vallee, R.B. Kinesin 3 and cytoplasmic dynein mediate interkinetic nuclear migration in neural stem cells. Nat. Neurosci. 13, 1463–1471 (2010).
Côté, J.F. & Vuori, K. Identification of an evolutionarily conserved superfamily of DOCK180-related proteins with guanine nucleotide exchange activity. J. Cell Sci. 115, 4901–4913 (2002).
Meller, N., Merlot, S. & Guda, C. CZH proteins: a new family of Rho-GEFs. J. Cell Sci. 118, 4937–4946 (2005).
Miyamoto, Y. & Yamauchi, J. Cellular signaling of Dock family proteins in neural function. Cell. Signal. 22, 175–182 (2010).
Laurin, M. et al. The atypical Rac activator Dock180 (Dock1) regulates myoblast fusion in vivo. Proc. Natl. Acad. Sci. USA 105, 15446–15451 (2008).
Fukui, Y. et al. Haematopoietic cell-specific CDM family protein DOCK2 is essential for lymphocyte migration. Nature 412, 826–831 (2001).
Chen, Q. et al. Loss of modifier of cell adhesion reveals a pathway leading to axonal degeneration. J. Neurosci. 29, 118–130 (2009).
Watabe-Uchida, M., John, K.A., Janas, J.A., Newey, S.E. & Van Aelst, L. The Rac activator DOCK7 regulates neuronal polarity through local phosphorylation of stathmin/Op18. Neuron 51, 727–739 (2006).
Yamauchi, J., Miyamoto, Y., Chan, J.R. & Tanoue, A. ErbB2 directly activates the exchange factor Dock7 to promote Schwann cell migration. J. Cell Biol. 181, 351–365 (2008).
Gergely, F. et al. The TACC domain identifies a family of centrosomal proteins that can interact with microtubules. Proc. Natl. Acad. Sci. USA 97, 14352–14357 (2000).
Peset, I. & Vernos, I. The TACC proteins: TACC-ling microtubule dynamics and centrosome function. Trends Cell Biol. 18, 379–388 (2008).
Blasius, A.L. et al. Mice with mutations of Dock7 have generalized hypopigmentation and white-spotting but show normal neurological function. Proc. Natl. Acad. Sci. USA 106, 2706–2711 (2009).
Minobe, S. et al. Rac is involved in the interkinetic nuclear migration of cortical progenitor cells. Neurosci. Res. 63, 294–301 (2009).
Liu, X., Hashimoto-Torii, K., Torii, M., Ding, C. & Rakic, P. Gap junctions/hemichannels modulate interkinetic nuclear migration in the forebrain precursors. J. Neurosci. 30, 4197–4209 (2010).
Janas, J., Skowronski, J. & Van Aelst, L. Lentiviral delivery of RNAi in hippocampal neurons. Methods Enzymol. 406, 593–605 (2006).
Kim, W.Y. et al. GSK-3 is a master regulator of neural progenitor homeostasis. Nat. Neurosci. 12, 1390–1397 (2009).
Shitamukai, A., Konno, D. & Matsuzaki, F. Oblique radial glial divisions in the developing mouse neocortex induce self-renewing progenitors outside the germinal zone that resemble primate outer subventricular zone progenitors. J. Neurosci. 31, 3683–3695 (2011).
Acknowledgements
We thank members of the Van Aelst laboratory, E.-E. Govek and J. Skowronski for discussions and/or critical reading of the manuscript. We thank N. Gray and J.W. Tsai for technical advice regarding the in utero electroporation procedure and K. John for yeast two-hybrid screening. We also thank F. Matsuzaki (RIKEN Center for Developmental Biology) and L.-H. Tsai (Massachusetts Institute of Technology) for reagents. This work was supported by US National Institutes of Health grant MH082808 and a New York STARR consortium grant to L.V.A. C.-L.W. is supported by US National Institutes of Health research training grant T32 CA 148056-1.
Author information
Authors and Affiliations
Contributions
Y.-T.Y., C.-L.W. and L.V.A. conceived and designed the project. Y.-T.Y. and C.-L.W. performed all the experiments and prepared the figures. L.V.A. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–12, Supplementary Data (PDF 6851 kb)
Supplementary Video 1
Example of a control vector expressing RGC (top left cell) undergoing bl-to-ap INM in VZ of neocortex. Time-lapse imaging was carried out on acute cortical slices 2 d after in utero electroporation of plasmids expressing empty control vector, and EGFP (green) and mKO2-F (red) fluorescent markers (at E15.5). Images acquired over an 8-h time period are shown; time (in min) is indicated on the top. Note: the cell body of the RGC migrates steadily toward the ventricle. After about 5 h, it reaches the ventricular (apical) surface, and the cell subsequently divides at the ventricular surface. (AVI 230 kb)
Supplementary Video 2
Example 1 of a DOCK7-overexpressing RGC (cell in the middle) undergoing bl-to-ap INM in VZ of neocortex. Time-lapse imaging was carried out on acute cortical slices 2 d after in utero electroporation of plasmids expressing FLAG-DOCK7, and EGFP (green) and mKO2-F (red) fluorescent markers (at E15.5). Images acquired over an 8-h time period are shown; time (in min) is indicated on the top. Note: the cell body of the RGC remains at its original basal position for about 5 h, which is followed by cell division away from the ventricular surface. (AVI 190 kb)
Supplementary Video 3
Example 2 of a DOCK7-overexpressing RGC (cell in upper middle) undergoing bl-to-ap INM in VZ of neocortex. Time-lapse imaging was carried out on acute cortical slices 2 d after in utero electroporation of plasmids expressing FLAG-DOCK7, and EGFP (green) and mKO2-F (red) fluorescent markers (at E15.5). Images acquired over an 8-h time period are shown; time (in min) is indicated on the top. Note: the cell body of the RGC remains at its basal position for about 4.5 h and then moves over a very short distance toward the ventricle, which is followed by cell division away from the ventricular surface. (AVI 235 kb)
Supplementary Video 4
Example of a control scr#1 shRNA–expressing RGC (cell in the middle) undergoing bl-to-ap INM in VZ of neocortex. Time-lapse imaging was carried out on acute cortical slices 2 d after in utero electroporation of plasmids expressing scr#1 shRNA, and EGFP (green) and mKO2-F (red) fluorescent markers (at E15.5). Images acquired over an 8-h time period are shown; time (in min) is indicated on the top. Note: the cell body of the RGC migrates steadily toward the ventricle. After about 5 h, it reaches the ventricular (apical) surface and the cell subsequently divides at the ventricular surface. (AVI 224 kb)
Supplementary Video 5
Example 1 of a Dock7#2 shRNA expressing RGC (cell in the middle) undergoing bl-to-ap INM in VZ of neocortex. Time-lapse imaging was carried out on acute cortical slices 2 d after in utero electroporation of plasmids expressing Dock7#2 shRNA, and EGFP (green) and mKO2-F (red) fluorescent markers (at E15.5). Images acquired over an 8-h time period are shown; time (in min) is indicated on the top. Note: the cell body of the RGC migrates considerably faster to the ventricle surface than that of the control scr#1 shRNA–expressing RGC, where it then remains for about 3 h before the cell undergoes apical mitosis. (AVI 204 kb)
Supplementary Video 6
Example 2 of a Dock7#2 shRNA expressing RGC (cell in the middle) undergoing bl-to-ap INM in VZ of neocortex. Time-lapse imaging was carried out on acute cortical slices 2 d after in utero electroporation of plasmids expressing Dock7#2 shRNA, and EGFP (green) and mKO2-F (red) fluorescent markers (at E15.5). Images acquired over an 8-h time period are shown; time (in min) is indicated on the top. Note: the cell body of the RGC migrates considerably faster to the ventricle surface than that of the control scr#1 shRNA expressing RGC, where it then remains for >4 h before the cell undergoes apical mitosis. (AVI 246 kb)
Rights and permissions
About this article
Cite this article
Yang, YT., Wang, CL. & Van Aelst, L. DOCK7 interacts with TACC3 to regulate interkinetic nuclear migration and cortical neurogenesis. Nat Neurosci 15, 1201–1210 (2012). https://doi.org/10.1038/nn.3171
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3171
This article is cited by
-
Chromatin-associated OGT promotes the malignant progression of hepatocellular carcinoma by activating ZNF263
Oncogene (2023)
-
Fusion of single-cell transcriptome and DNA-binding data, for genomic network inference in cortical development
BMC Bioinformatics (2021)
-
18q22.1-qter deletion and 4p16.3 microduplication in a boy with speech delay and mental retardation: case report and review of the literature
Molecular Cytogenetics (2018)
-
Association between the DOCK7, PCSK9 and GALNT2 Gene Polymorphisms and Serum Lipid levels
Scientific Reports (2016)
-
Microtubule plus-end tracking proteins in neuronal development
Cellular and Molecular Life Sciences (2016)