Abstract
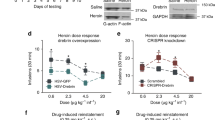
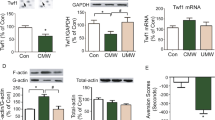
Repeated cocaine administration increases the dendritic arborization of nucleus accumbens neurons, but the underlying signaling events remain unknown. Here we show that repeated exposure to cocaine negatively regulates the active form of Rac1, a small GTPase that controls actin remodeling in other systems. Further, we show, using viral-mediated gene transfer, that overexpression of a dominant negative mutant of Rac1 or local knockout of Rac1 is sufficient to increase the density of immature dendritic spines on nucleus accumbens neurons, whereas overexpression of a constitutively active Rac1 or light activation of a photoactivatable form of Rac1 blocks the ability of repeated cocaine exposure to produce this effect. Downregulation of Rac1 activity likewise promotes behavioral responses to cocaine exposure, with activation of Rac1 producing the opposite effect. These findings establish that Rac1 signaling mediates structural and behavioral plasticity in response to cocaine exposure.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Garey, L.J. et al. Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. J. Neurol. Neurosurg. Psychiatry 65, 446–453 (1998).
Russo, S.J. et al. The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci. 33, 267–276 (2010).
Soetanto, A. et al. Association of anxiety and depression with microtubule-associated protein 2- and synaptopodin-immunolabeled dendrite and spine densities in hippocampal CA3 of older humans. Arch. Gen. Psychiatry 67, 448–457 (2010).
Hyman, S.E., Malenka, R.C. & Nestler, E.J. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu. Rev. Neurosci. 29, 565–598 (2006).
Robinson, T.E. & Kolb, B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology 47, 33–46 (2004).
Kalivas, P.W. The glutamate homeostasis hypothesis of addiction. Nat. Rev. Neurosci. 10, 561–572 (2009).
Nimchinsky, E.A., Sabatini, B.L. & Svoboda, K. Structure and function of dendritic spines. Annu. Rev. Physiol. 64, 313–353 (2002).
Deng, J.V. et al. MeCP2 in the nucleus accumbens contributes to neural and behavioral responses to psychostimulants. Nat. Neurosci. 13, 1128–1136 (2010).
LaPlant, Q. et al. Dnmt3a regulates emotional behavior and spine plasticity in the nucleus accumbens. Nat. Neurosci. 13, 1137–1143 (2010).
Maze, I. et al. Essential role of the histone methyltransferase G9a in cocaine-induced plasticity. Science 327, 213–216 (2010).
Norrholm, S.D. et al. Cocaine-induced proliferation of dendritic spines in nucleus accumbens is dependent on the activity of cyclin-dependent kinase-5. Neuroscience 116, 19–22 (2003).
Pulipparacharuvil, S. et al. Cocaine regulates MEF2 to control synaptic and behavioral plasticity. Neuron 59, 621–633 (2008).
Russo, S.J. et al. Nuclear factor kappa B signaling regulates neuronal morphology and cocaine reward. J. Neurosci. 29, 3529–3537 (2009).
Trachtenberg, J.T. et al. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature 420, 788–794 (2002).
Holtmaat, A. & Svoboda, K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat. Rev. Neurosci. 10, 647–658 (2009).
Toda, S., Shen, H.W., Peters, J., Cagle, S. & Kalivas, P.W. Cocaine increases actin cycling: effects in the reinstatement model of drug seeking. J. Neurosci. 26, 1579–1587 (2006).
Shen, H.W. et al. Altered dendritic spine plasticity in cocaine-withdrawn rats. J. Neurosci. 29, 2876–2884 (2009).
Toda, S., Shen, H. & Kalivas, P.W. Inhibition of actin polymerization prevents cocaine-induced changes in spine morphology in the nucleus accumbens. Neurotox. Res. 18, 410–415 (2010).
Halpain, S. Actin and the agile spine: how and why do dendritic spines dance? Trends Neurosci. 23, 141–146 (2000).
Penzes, P. & Jones, K.A. Dendritic spine dynamics—a key role for kalirin-7. Trends Neurosci. 31, 419–427 (2008).
Hayashi-Takagi, A. et al. Disrupted-in-Schizophrenia 1 (DISC1) regulates spines of the glutamate synapse via Rac1. Nat. Neurosci. 13, 327–332 (2010).
Tashiro, A., Minden, A. & Yuste, R. Regulation of dendritic spine morphology by the rho family of small GTPases: antagonistic roles of Rac and Rho. Cereb. Cortex 10, 927–938 (2000).
Tashiro, A. & Yuste, R. Regulation of dendritic spine motility and stability by Rac1 and Rho kinase: evidence for two forms of spine motility. Mol. Cell Neurosci. 26, 429–440 (2004).
Oh, D. et al. Regulation of synaptic Rac1 activity, long-term potentiation maintenance, and learning and memory by BCR and ABR Rac GTPase-activating proteins. J. Neurosci. 30, 14134–14144 (2010).
Luo, L. et al. Differential effects of the Rac GTPase on Purkinje cell axons and dendritic trunks and spines. Nature 379, 837–840 (1996).
Yang, N. et al. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature 393, 809–812 (1998).
Edwards, D.C., Sanders, L.C., Bokoch, G.M. & Gill, G.N. Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nat. Cell Biol. 1, 253–259 (1999).
Shirazi Fard, S., Kele, J., Vilar, M., Paratcha, G. & Ledda, F. Tiam1 as a signaling mediator of nerve growth factor-dependent neurite outgrowth. PLoS ONE 5, e9647 (2010).
Miyamoto, Y., Yamauchi, J., Tanoue, A., Wu, C. & Mobley, W.C. TrkB binds and tyrosine-phosphorylates Tiam1, leading to activation of Rac1 and induction of changes in cellular morphology. Proc. Natl. Acad. Sci. USA 103, 10444–10449 (2006).
Nobes, C.D. & Hall, A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J. Cell Biol. 144, 1235–1244 (1999).
Marinissen, M.J. et al. The small GTP-binding protein RhoA regulates c-Jun by a ROCK-JNK signaling axis. Mol. Cell 14, 29–41 (2004).
Chen, L., Melendez, J., Campbell, K., Kuan, C.Y. & Zheng, Y. Rac1 deficiency in the forebrain results in neural progenitor reduction and microcephaly. Dev. Biol. 325, 162–170 (2009).
Gu, Y. et al. Hematopoietic cell regulation by Rac1 and Rac2 guanosine triphosphatases. Science 302, 445–449 (2003).
Wu, Y.I. et al. A genetically encoded photoactivatable Rac controls the motility of living cells. Nature 461, 104–108 (2009).
Lobo, M.K. et al. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science 330, 385–390 (2010).
Kim, W.Y., Shin, S.R., Kim, S., Jeon, S. & Kim, J.H. Cocaine regulates ezrin-radixin-moesin proteins and RhoA signaling in the nucleus accumbens. Neuroscience 163, 501–505 (2009).
Hering, H. & Sheng, M. Dendritic spines: structure, dynamics and regulation. Nat. Rev. Neurosci. 2, 880–888 (2001).
Ghosh, M. et al. Cofilin promotes actin polymerization and defines the direction of cell motility. Science 304, 743–746 (2004).
Bosco, E.E., Mulloy, J.C. & Zheng, Y. Rac1 GTPase: a “Rac” of all trades. Cell Mol. Life Sci. 66, 370–374 (2009).
Kalivas, P.W., Volkow, N. & Seamans, J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron 45, 647–650 (2005).
Schmidt, H.D. & Pierce, R.C. Cocaine-induced neuroadaptations in glutamate transmission: potential therapeutic targets for craving and addiction. Ann. NY Acad. Sci. 1187, 35–75 (2010).
Thomas, M.J., Kalivas, P.W. & Shaham, Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br. J. Pharmacol. 154, 327–342 (2008).
Wolf, M.E. The Bermuda Triangle of cocaine-induced neuroadaptations. Trends Neurosci. 33, 391–398 (2010).
Thomas, M.J., Beurrier, C., Bonci, A. & Malenka, R.C. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nat. Neurosci. 4, 1217–1223 (2001).
Huang, Y.H. et al. In vivo cocaine experience generates silent synapses. Neuron 63, 40–47 (2009).
Kiraly, D.D. et al. Behavioral and morphological responses to cocaine require Kalirin7. Biol. Psychiatry 68, 249–255 (2010).
Chen, B.T. et al. Cocaine but not natural reward self-administration nor passive cocaine infusion produces persistent LTP in the VTA. Neuron 59, 288–297 (2008).
McCutcheon, J.E., Wang, X., Tseng, K.Y., Wolf, M.E. & Marinelli, M. Calcium-permeable AMPA receptors are present in nucleus accumbens synapses after prolonged withdrawal from cocaine self-administration but not experimenter-administered cocaine. J. Neurosci. 31, 5737–5743 (2011).
McFarland, K., Lapish, C.C. & Kalivas, P.W. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J. Neurosci. 23, 3531–3537 (2003).
Barrot, M. et al. CREB activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli. Proc. Natl. Acad. Sci. USA 99, 11435–11440 (2002).
Acknowledgements
We thank S. Golden, A. Robison and V. Vialou for helpful discussions and comments on the manuscript. This work was supported by grants from the US National Institute on Drug Abuse (R01 DA14133, P01 DA08227) and US National Institute of Mental Health (R01 MH51399).
Author information
Authors and Affiliations
Contributions
D.M.D., S.J.R. and E.J.N. were responsible for overall study design. D.M.D., H.S., K.C.D., C.D. and I.M. designed and conducted GTPase activity assays and analyzed the data. D.M.D., M.E.C., J.W.K. and D.F. carried out the stereotaxic surgeries and behavioral experiments. D.M.D., M.S.M.-R., D.D.-W., V.G. and H.S. carried out and analyzed the western blots. D.M.D., D.C. and V.G. scanned, counted and analyzed the spine data. D.M.D., M.K.L., H.S., K.N.S., G.E.H., S.J.R., Y.O. and K.M.H. designed and did the necessary cloning and conducted the optical Rac1-pa experiments. Y.Z. provided the floxed Rac1 mice and expertise in Rac1 signaling; R.L.N. constructed and provided the viral vectors for gene transfer. D.M.D. and E.J.N. wrote the paper with the help of the other authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3 (PDF 2164 kb)
Rights and permissions
About this article
Cite this article
Dietz, D., Sun, H., Lobo, M. et al. Rac1 is essential in cocaine-induced structural plasticity of nucleus accumbens neurons. Nat Neurosci 15, 891–896 (2012). https://doi.org/10.1038/nn.3094
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3094
This article is cited by
-
Methamphetamine-induced region-specific transcriptomic and epigenetic changes in the brain of male rats
Communications Biology (2023)
-
Astrocytes in cocaine addiction and beyond
Molecular Psychiatry (2022)
-
Interactions of neuroimmune signaling and glutamate plasticity in addiction
Journal of Neuroinflammation (2021)
-
Mice carrying a schizophrenia-associated mutation of the Arhgap10 gene are vulnerable to the effects of methamphetamine treatment on cognitive function: association with morphological abnormalities in striatal neurons
Molecular Brain (2021)
-
Silent synapses dictate cocaine memory destabilization and reconsolidation
Nature Neuroscience (2020)