Abstract
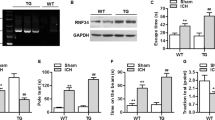
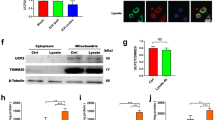
Phagocytic cell NADPH oxidase (NOX) generates reactive oxygen species (ROS) as part of innate immunity. Unfortunately, ischemia can also induce this pathway and inflict damage on native cells. The voltage-gated proton channel Hv1 enables NOX function by compensating cellular loss of electrons with protons. Accordingly, we investigated whether NOX-mediated brain damage in stroke can be inhibited by suppression of Hv1. We found that mouse and human brain microglia, but not neurons or astrocytes, expressed large Hv1-mediated currents. Hv1 was required for NOX-dependent ROS generation in brain microglia in situ and in vivo. Mice lacking Hv1 were protected from NOX-mediated neuronal death and brain damage 24 h after stroke. These results indicate that Hv1-dependent ROS production is responsible for a substantial fraction of brain damage at early time points after ischemic stroke and provide a rationale for Hv1 as a therapeutic target for the treatment of ischemic stroke.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Nathan, C. & Ding, A. SnapShot: reactive oxygen intermediates (ROI). Cell 140, 951 (2010).
Steinhubl, S.R. Why have antioxidants failed in clinical trials? Am. J. Cardiol. 101, 14D–19D (2008).
Bedard, K. & Krause, K.H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 87, 245–313 (2007).
Dringen, R. Oxidative and antioxidative potential of brain microglial cells. Antioxid. Redox Signal. 7, 1223–1233 (2005).
Walder, C.E. et al. Ischemic stroke injury is reduced in mice lacking a functional NADPH oxidase. Stroke 28, 2252–2258 (1997).
Kleinschnitz, C. et al. Post-stroke inhibition of induced NADPH oxidase type 4 prevents oxidative stress and neurodegeneration. PLoS Biol. 8, e1000479 (2010).
Decoursey, T.E. Voltage-gated proton channels and other proton transfer pathways. Physiol. Rev. 83, 475–579 (2003).
Decoursey, T.E., Morgan, D. & Cherny, V.V. The voltage dependence of NADPH oxidase reveals why phagocytes need proton channels. Nature 422, 531–534 (2003).
Ramsey, I.S., Moran, M.M., Chong, J.A. & Clapham, D.E. A voltage-gated proton-selective channel lacking the pore domain. Nature 440, 1213–1216 (2006).
Sasaki, M., Takagi, M. & Okamura, Y. A voltage sensor-domain protein is a voltage-gated proton channel. Science 312, 589–592 (2006).
Okochi, Y., Sasaki, M., Iwasaki, H. & Okamura, Y. Voltage-gated proton channel is expressed on phagosomes. Biochem. Biophys. Res. Commun. 382, 274–279 (2009).
Ramsey, I.S., Ruchti, E., Kaczmarek, J.S. & Clapham, D.E. Hv1 proton channels are required for high-level NADPH oxidase–dependent superoxide production during the phagocyte respiratory burst. Proc. Natl. Acad. Sci. USA 106, 7642–7647 (2009).
Capasso, M. et al. HVCN1 modulates BCR signal strength via regulation of BCR-dependent generation of reactive oxygen species. Nat. Immunol. 11, 265–272 (2010).
Musset, B. et al. A pH-stabilizing role of voltage-gated proton channels in IgE-mediated activation of human basophils. Proc. Natl. Acad. Sci. USA 105, 11020–11025 (2008).
Iovannisci, D., Illek, B. & Fischer, H. Function of the HVCN1 proton channel in airway epithelia and a naturally occurring mutation, M91T. J. Gen. Physiol. 136, 35–46 (2010).
Lishko, P.V., Botchkina, I.L., Fedorenko, A. & Kirichok, Y. Acid extrusion from human spermatozoa is mediated by flagellar voltage-gated proton channel. Cell 140, 327–337 (2010).
Thomas, R.C. & Meech, R.W. Hydrogen ion currents and intracellular pH in depolarized voltage-clamped snail neurones. Nature 299, 826–828 (1982).
De Simoni, A., Allen, N.J. & Attwell, D. Charge compensation for NADPH oxidase activity in microglia in rat brain slices does not involve a proton current. Eur. J. Neurosci. 28, 1146–1156 (2008).
Schilling, T. & Eder, C. Ion channel expression in resting and activated microglia of hippocampal slices from juvenile mice. Brain Res. 1186, 21–28 (2007).
DeCoursey, T.E. Voltage-gated proton channels: what's next? J. Physiol. (Lond.) 586, 5305–5324 (2008).
Visentin, S., Agresti, C., Patrizio, M. & Levi, G. Ion channels in rat microglia and their different sensitivity to lipopolysaccharide and interferon-gamma. J. Neurosci. Res. 42, 439–451 (1995).
Cheng, Y.M., Kelly, T. & Church, J. Potential contribution of a voltage-activated proton conductance to acid extrusion from rat hippocampal neurons. Neuroscience 151, 1084–1098 (2008).
Boron, W.F. & De Weer, P. Intracellular pH transients in squid giant axons caused by CO2, NH3 and metabolic inhibitors. J. Gen. Physiol. 67, 91–112 (1976).
Davalos, D. et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 8, 752–758 (2005).
Wu, L.J., Vadakkan, K.I. & Zhuo, M. ATP-induced chemotaxis of microglial processes requires P2Y receptor–activated initiation of outward potassium currents. Glia 55, 810–821 (2007).
Block, M.L., Zecca, L. & Hong, J.S. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat. Rev. Neurosci. 8, 57–69 (2007).
Gambhir, S.S. et al. A tabulated summary of the FDG PET literature. J. Nucl. Med. 42, 1S–93S (2001).
Lyons, S.A. et al. Distinct physiologic properties of microglia and blood-borne cells in rat brain slices after permanent middle cerebral artery occlusion. J. Cereb. Blood Flow Metab. 20, 1537–1549 (2000).
Kraft, R. et al. Hydrogen peroxide and ADP-ribose induce TRPM2-mediated calcium influx and cation currents in microglia. Am. J. Physiol. Cell Physiol. 286, C129–C137 (2004).
Whittingham, T.S., Lust, W.D. & Passonneau, J.V. An in vitro model of ischemia: metabolic and electrical alterations in the hippocampal slice. J. Neurosci. 4, 793–802 (1984).
Brennan, A.M. et al. NADPH oxidase is the primary source of superoxide induced by NMDA receptor activation. Nat. Neurosci. 12, 857–863 (2009).
Chan, P.H. Reactive oxygen radicals in signaling and damage in the ischemic brain. J. Cereb. Blood Flow Metab. 21, 2–14 (2001).
Lai, A.Y. & Todd, K.G. Microglia in cerebral ischemia: molecular actions and interactions. Can. J. Physiol. Pharmacol. 84, 49–59 (2006).
Yenari, M.A., Kauppinen, T.M. & Swanson, R.A. Microglial activation in stroke: therapeutic targets. Neurotherapeutics 7, 378–391 (2010).
Liu, R. et al. Reversal of age-related learning deficits and brain oxidative stress in mice with superoxide dismutase/catalase mimetics. Proc. Natl. Acad. Sci. USA 100, 8526–8531 (2003).
Jin, R., Yang, G. & Li, G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J. Leukoc. Biol. 87, 779–789 (2010).
Lalancette-Hébert, M., Gowing, G., Simard, A., Weng, Y.C. & Kriz, J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J. Neurosci. 27, 2596–2605 (2007).
Sorce, S. & Krause, K.H. NOX enzymes in the central nervous system: from signaling to disease. Antioxid. Redox Signal. 11, 2481–2504 (2009).
Schilling, M. et al. Microglial activation precedes and predominates over macrophage infiltration in transient focal cerebral ischemia: a study in green fluorescent protein transgenic bone marrow chimeric mice. Exp. Neurol. 183, 25–33 (2003).
Gelderblom, M. et al. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke 40, 1849–1857 (2009).
Ransohoff, R.M. Microgliosis: the questions shape the answers. Nat. Neurosci. 10, 1507–1509 (2007).
Iadecola, C. & Anrather, J. The immunology of stroke: from mechanisms to translation. Nat. Med. 17, 796–808 (2011).
Moskowitz, M.A., Lo, E.H. & Iadecola, C. The science of stroke: mechanisms in search of treatments. Neuron 67, 181–198 (2010).
Jung, S. et al. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell. Biol. 20, 4106–4114 (2000).
Wu, L.J. & Zhuo, M. Resting microglial motility is independent of synaptic plasticity in mammalian brain. J. Neurophysiol. 99, 2026–2032 (2008).
Longa, E.Z., Weinstein, P.R., Carlson, S. & Cummins, R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20, 84–91 (1989).
Fisher, M. et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke 40, 2244–2250 (2009).
Hara, H., Huang, P.L., Panahian, N., Fishman, M.C. & Moskowitz, M.A. Reduced brain edema and infarction volume in mice lacking the neuronal isoform of nitric oxide synthase after transient MCA occlusion. J. Cereb. Blood Flow Metab. 16, 605–611 (1996).
Zhao, H. et al. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic. Biol. Med. 34, 1359–1368 (2003).
Boyarsky, G., Ganz, M.B., Sterzel, R.B. & Boron, W.F. pH regulation in single glomerular mesangial cells. I. Acid extrusion in absence and presence of HCO3. Am. J. Physiol. 255, C844–C856 (1988).
Acknowledgements
We thank L. Silberstein, W. Yang and M. Kurtev for technical assistance, S. Doctrow (Boston University) for kindly providing us with EUK-207, Y. Kirichok (University of California, San Francisco) for Hv1 antibody, and D. Chaudhuri for critical reading of the manuscript. This work was supported by the US National Institutes of Health (R01 MH090293 to D.E.C. and R01 EY019029 to E.P.F.). L.-J.W. is supported by a Lefler postdoctoral fellowship from Harvard Medical School and a Scientist Development Grant from the American Heart Association (11SDG7340011).
Author information
Authors and Affiliations
Contributions
L.-J.W. performed experiments, including electrophysiology, imaging, immunostaining, cell culture, quantitative RT-PCR, western blot and the mouse stroke models. G.W. performed the mouse stroke models, and western blot, immunostaining, quantitative RT-PCR and cell culture experiments. M.R.A.S. conducted MRI experiments. A.B. conducted PET and computed tomography imaging experiments. Y.J conducted bone marrow transplantation experiments. L.-J.W., G.W., Y.J., F.H.F. and D.E.C. conducted the data analyses. L.-J.W. and D.E.C. wrote the manuscript. L.-J.W., H.R.L., E.P.F. and D.E.C. supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–14 (PDF 1238 kb)
Supplementary Movie 1
Time-lapse imaging of increased ROS production in wt and Hv1−/− microglia in brain slices after PMA perfusion (10s/frame) (AVI 4333 kb)
Supplementary Movie 2
Time-lapse imaging of ATP-induced chemotaxis in wt and Hv1−/− microglia in brain slices (1 min/frame) (AVI 3686 kb)
Supplementary Movie 3
MRI images of coronal section of whole brain 24 hours after pMCAO (AVI 1156 kb)
Rights and permissions
About this article
Cite this article
Wu, LJ., Wu, G., Sharif, M. et al. The voltage-gated proton channel Hv1 enhances brain damage from ischemic stroke. Nat Neurosci 15, 565–573 (2012). https://doi.org/10.1038/nn.3059
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3059
This article is cited by
-
Role of the Voltage-Gated Proton Channel Hv1 in Nervous Systems
Neuroscience Bulletin (2023)
-
Chemogenetic and Optogenetic Manipulations of Microglia in Chronic Pain
Neuroscience Bulletin (2023)
-
Ion currents through the voltage sensor domain of distinct families of proteins
Journal of Biological Physics (2023)
-
Targeting Hv1 proton channel for pain control
Cell Research (2022)
-
Inhibiting Hv1 channel in peripheral sensory neurons attenuates chronic inflammatory pain and opioid side effects
Cell Research (2022)