Abstract
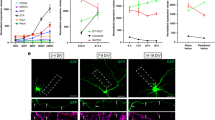
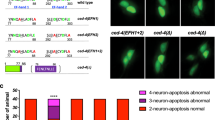
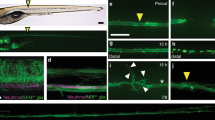
The ability of neurons to undergo regenerative growth after injury is governed by cell-intrinsic and cell-extrinsic regeneration pathways. These pathways represent potential targets for therapies to enhance regeneration. However, the signaling pathways that orchestrate axon regeneration are not well understood. In Caenorhabditis elegans, the Jun N-terminal kinase (JNK) and p38 MAP kinase (MAPK) pathways are important for axon regeneration. We found that the C. elegans SVH-1 growth factor and its receptor, SVH-2 tyrosine kinase, regulate axon regeneration. Loss of SVH-1–SVH-2 signaling resulted in a substantial defect in the ability of neurons to regenerate, whereas its activation improved regeneration. Furthermore, SVH-1–SVH-2 signaling was initiated extrinsically by a pair of sensory neurons and functioned upstream of the JNK-MAPK pathway. Thus, SVH-1–SVH-2 signaling via activation of the MAPK pathway acts to coordinate neuron regeneration response after axon injury.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Yanik, M.F. et al. Neurosurgery: functional regeneration after laser axotomy. Nature 432, 822 (2004).
Hammarlund, M., Jorgensen, E.M. & Bastiani, M.J. Axons break in animals lacking beta-spectrin. J. Cell Biol. 176, 269–275 (2007).
O'Brien, G.S. & Sagasti, A. Fragile axons forge the path to gene discovery: a MAP kinase pathway regulates axon regeneration. Sci. Signal. 2, e30 (2009).
Hammarlund, M., Nix, P., Hauth, L., Jorgensen, E.M. & Bastiani, M. Axon regeneration requires a conserved MAP kinase pathway. Science 323, 802–806 (2009).
Yan, D., Wu, Z., Chisholm, A.D. & Jin, Y. The DLK-1 kinase promotes mRNA stability and local translation in C. elegans synapses and axon regeneration. Cell 138, 1005–1018 (2009).
Nix, P., Hisamoto, N., Matsumoto, K. & Bastiani, M. Axon regeneration requires co-activation of p38 and JNK MAPK pathways. Proc. Natl. Acad. Sci. USA 108, 10738–10743 (2011).
Camps, M., Nichols, A. & Arkinstall, S. Dual specificity phosphatases: a gene family for control of MAP kinase function. FASEB J. 14, 6–16 (2000).
Mizuno, T. et al. The Caenorhabditis elegans MAPK phosphatase VHP-1 mediates a novel JNK-like signalling pathway in stress response. EMBO J. 23, 2226–2234 (2004).
Donate, L.E. et al. Molecular evolution and domain structure of plasminogen-related growth factors (HGF/SF and HGFI/MSP). Protein Sci. 3, 2378–2394 (1994).
Trusolino, L., Bertotti, A. & Comoglio, P.M. MET signalling: principles and functions in development, organ regeneration and cancer. Nat. Rev. Mol. Cell Biol. 11, 834–848 (2010).
Gaudino, G. et al. RON is a heterodimeric tyrosine kinase receptor activated by the HGF homologue MSP. EMBO J. 13, 3524–3532 (1994).
Hart, A.C., Sims, S. & Kaplan, J.M. Synaptic code for sensory modalities revealed by C. elegans GLR-1 glutamate receptor. Nature 378, 82–85 (1995).
McCarroll, S.A., Li, H. & Bargmann, C.I. Identification of transcriptional regulatory elements in chemosensory receptor genes by probabilistic segmentation. Curr. Biol. 15, 347–352 (2005).
Rodrigues, G.A. & Park, M. Dimerization mediated through a leucine zipper activates the oncogenic potential of the met receptor tyrosine kinase. Mol. Cell Biol. 13, 6711–6722 (1993).
Santoro, M.M., Collesi, C., Grisendi, S., Gaudino, G. & Comoglio, P.M. Constitutive activation of the RON gene promotes invasive growth but not transformation. Mol. Cell Biol. 16, 7072–7083 (1996).
Ferracini, R. et al. Identification of the major autophosphorylation site of the Met/hepatocyte growth factor receptor tyrosine kinase. J. Biol. Chem. 266, 19558–19564 (1991).
Knobel, K.M., Jorgensen, E.M. & Bastiani, M.J. Growth cones stall and collapse during axon outgrowth in Caenorhabditis elegans. Development 126, 4489–4498 (1999).
Nakata, K. et al. Regulation of a DLK-1 and p38 MAP kinase pathway by the ubiquitin ligase RPM-1 is required for presynaptic development. Cell 120, 407–420 (2005).
Zhen, M., Huang, X., Bamber, B. & Jin, Y. Regulation of presynaptic terminal organization by C. elegans RPM-1, a putative guanine nucleotide exchanger with a RING-H2 finger domain. Neuron 26, 331–343 (2000).
Schaefer, A.M., Hadwiger, G.D. & Nonet, M.L. rpm-1, a conserved neuronal gene that regulates targeting and synaptogenesis in C. elegans. Neuron 26, 345–356 (2000).
Mizuno, T., Fujiki, K., Sasakawa, A., Hisamoto, N. & Matsumoto, K. Role of the Caenorhabditis elegans Shc adaptor protein in the c-Jun N-terminal kinase signalling pathway. Mol. Cell Biol. 28, 7041–7049 (2008).
Brenner, S. The genetics of Caenorhabditis elegans. Genetics 77, 71–94 (1974).
Kamath, R.K., Martinez-Campos, M., Zipperlen, P., Fraser, A.G. & Ahringer, J. Effectiveness of specific RNA-mediated interference through ingested double-stranded RNA in C. elegans. Genome Biol. 2, 1–10 (2001).
Fang-Yen, C., Gabel, C.V., Samuel, A.D., Bargmann, C.I. & Avery, J. Laser microsurgery in Caenorhabditis elegans. Methods Cell Biol. 107, 177–206 (2012).
Sakamoto, R. et al. The Caenorhabditis elegans UNC-14 RUN domain protein binds to the kinesin-1 and UNC-16 complex and regulates synaptic vesicle localization. Mol. Biol. Cell 16, 483–496 (2005).
Arimoto, M. et al. The Caenorhabditis elegans JIP3 protein UNC-16 functions as an adaptor to link kinesin-1 with cytoplasmic dynein. J. Neurosci. 31, 2216–2224 (2011).
Colicelli, J. et al. Expression of three mammalian cDNAs that interfere with RAS function in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 88, 2913–2917 (1991).
Fujiki, K., Mizuno, T., Hisamoto, N. & Matsumoto, K. The Caenorhabditis elegans Ste20 kinase and Rac-type small GTPase regulate the c-Jun N-terminal kinase signalling pathway mediating the stress response. Mol. Cell Biol. 30, 995–1003 (2010).
Mello, C.C., Kramer, J.M., Stinchcomb, D. & Ambros, V. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 10, 3959–3970 (1991).
Bork, P., Doerks, T., Springer, T.A. & Snel, B. Domains in plexins: links to integrins and transcription factors. Trends Biochem. Sci. 24, 261–263 (1999).
Acknowledgements
We thank Y. Kohara, S. Mitani, the Caenorhabditis Genetic Center and the C. elegans Knockout Consortium for materials. This work was supported by grants from Ministry of Education, Culture and Science of Japan, the Sumitomo Foundation (to K.M., T.M. and N.H.), the Nakajima Science Foundation (to T.M.), the National Science Foundation, the McKnight Endowment Fund for Neuroscience, the Christopher and Dana Reeve Foundation, and the Amerisure Charitable Foundation (to M.B. and P.N.). C.L. was supported by a Japan Society for the Promotion of Science Research Fellowship.
Author information
Authors and Affiliations
Contributions
C.L., N.H., P.N., M.B. and K.M. designed the experiments and analyzed data. S.K. and T.M. performed the biochemical experiments. P.N. and M.B. carried out the time-lapse experiments. C.L. and N.H. performed all of the other experiments. The manuscript was written by K.M. and commented on by N.H., P.N. and M.B.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 and Supplementary Tables 1 and 2 (PDF 9979 kb)
Supplementary Video 1
Time-lapse image of severed axon of D-type motor neuron in wild-type animal. (MOV 11031 kb)
Supplementary Video 2
Time-lapse image of severed axon of D-type motor neuron in svh-2 mutant animal. (MOV 1558 kb)
Supplementary Video 3
Time-lapse image of severed axon of D-type motor neuron in svh-2 mutant animal. (MOV 2953 kb)
Supplementary Video 4
Time-lapse image of severed axon of D-type motor neuron in svh-1 mutant animal. (MOV 453 kb)
Supplementary Video 5
Time-lapse image of severed axon of D-type motor neuron in svh-1-overexpressing animal. (MOV 10209 kb)
Supplementary Video 6
Time-lapse image of severed axon of D-type motor neuron in svh-2-overexpressing animal. (MOV 8468 kb)
Rights and permissions
About this article
Cite this article
Li, C., Hisamoto, N., Nix, P. et al. The growth factor SVH-1 regulates axon regeneration in C. elegans via the JNK MAPK cascade. Nat Neurosci 15, 551–557 (2012). https://doi.org/10.1038/nn.3052
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3052
This article is cited by
-
Molecular mechanisms of neurite regeneration and repair: insights from C. elegans and Drosophila
Cell Regeneration (2023)
-
Femtosecond laser microdissection for isolation of regenerating C. elegans neurons for single-cell RNA sequencing
Nature Methods (2023)
-
Phosphatidylserine exposure mediated by ABC transporter activates the integrin signaling pathway promoting axon regeneration
Nature Communications (2018)
-
Intrinsic JNK-MAPK pathway involvement requires daf-16-mediated immune response during Shigella flexneri infection in C. elegans
Immunologic Research (2017)
-
Axotomy-induced HIF-serotonin signalling axis promotes axon regeneration in C. elegans
Nature Communications (2016)