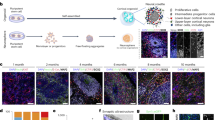
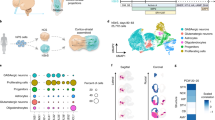
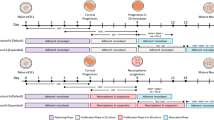
Abstract
Efforts to study the development and function of the human cerebral cortex in health and disease have been limited by the availability of model systems. Extrapolating from our understanding of rodent cortical development, we have developed a robust, multistep process for human cortical development from pluripotent stem cells: directed differentiation of human embryonic stem (ES) and induced pluripotent stem (iPS) cells to cortical stem and progenitor cells, followed by an extended period of cortical neurogenesis, neuronal terminal differentiation to acquire mature electrophysiological properties, and functional excitatory synaptic network formation. We found that induction of cortical neuroepithelial stem cells from human ES cells and human iPS cells was dependent on retinoid signaling. Furthermore, human ES cell and iPS cell differentiation to cerebral cortex recapitulated in vivo development to generate all classes of cortical projection neurons in a fixed temporal order. This system enables functional studies of human cerebral cortex development and the generation of individual-specific cortical networks ex vivo for disease modeling and therapeutic purposes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Mountcastle, V.B. The Cerebral Cortex (Harvard University Press, Cambridge, Massachusetts, 1998).
Finlay, B.L. & Darlington, R.B. Linked regularities in the development and evolution of mammalian brains. Science 268, 1578–1584 (1995).
Rakic, P. Evolution of the neocortex: a perspective from developmental biology. Nat. Rev. Neurosci. 10, 724–735 (2009).
Hill, R.S. & Walsh, C.A. Molecular insights into human brain evolution. Nature 437, 64–67 (2005).
Wonders, C.P. & Anderson, S.A. The origin and specification of cortical interneurons. Nat. Rev. Neurosci. 7, 687–696 (2006).
Caviness, V.S. Jr., Takahashi, T. & Nowakowski, R.S. Numbers, time and neocortical neuronogenesis: a general developmental and evolutionary model. Trends Neurosci. 18, 379–383 (1995).
Douglas, R.J. & Martin, K.A. Neuronal circuits of the neocortex. Annu. Rev. Neurosci. 27, 419–451 (2004).
Fame, R.M., MacDonald, J.L. & Macklis, J.D. Development, specification, and diversity of callosal projection neurons. Trends Neurosci. 34, 41–50 (2011).
López-Bendito, G. & Molnar, Z. Thalamocortical development: how are we going to get there? Nat. Rev. Neurosci. 4, 276–289 (2003).
Bibel, M. et al. Differentiation of mouse embryonic stem cells into a defined neuronal lineage. Nat. Neurosci. 7, 1003–1009 (2004).
Gaspard, N. et al. An intrinsic mechanism of corticogenesis from embryonic stem cells. Nature 455, 351–357 (2008).
Eiraku, M. et al. Self-organized formation of polarized cortical tissues from ES cells and its active manipulation by extrinsic signals. Cell Stem Cell 3, 519–532 (2008).
Au, E. & Fishell, G. Cortex shatters the glass ceiling. Cell Stem Cell 3, 472–474 (2008).
Hansen, D.V., Rubenstein, J.L. & Kriegstein, A.R. Deriving excitatory neurons of the neocortex from pluripotent stem cells. Neuron 70, 645–660 (2011).
Hansen, D.V., Lui, J.H., Parker, P.R. & Kriegstein, A.R. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature 464, 554–561 (2010).
Wang, X., Tsai, J.W., Lamonica, B. & Kriegstein, A.R. A new subtype of progenitor cell in the mouse embryonic neocortex. Nat. Neurosci. 14, 555–561 (2011).
Fietz, S.A. et al. OSVZ progenitors of human and ferret neocortex are epithelial-like and expand by integrin signaling. Nat. Neurosci. 13, 690–699 (2010).
Ying, Q.L., Stavridis, M., Griffiths, D., Li, M. & Smith, A. Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat. Biotechnol. 21, 183–186 (2003).
Chambers, S.M. et al. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 27, 275–280 (2009).
Hu, B.Y. & Zhang, S.C. Directed differentiation of neural-stem cells and subtype-specific neurons from human ES cells. Methods Mol. Biol. 636, 123–137 (2010).
Elkabetz, Y. et al. Human ES cell–derived neural rosettes reveal a functionally distinct early neural stem cell stage. Genes Dev. 22, 152–165 (2008).
Zhang, S.C., Wernig, M., Duncan, I.D., Brustle, O. & Thomson, J.A. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat. Biotechnol. 19, 1129–1133 (2001).
Götz, M. & Huttner, W.B. The cell biology of neurogenesis. Nat. Rev. Mol. Cell Biol. 6, 777–788 (2005).
Fietz, S.A. & Huttner, W.B. Cortical progenitor expansion, self-renewal and neurogenesis: a polarized perspective. Curr. Opin. Neurobiol. 21, 23–35 (2011).
Takahashi, T., Nowakowski, R.S. & Caviness, V.S. Jr. The leaving or Q fraction of the murine cerebral proliferative epithelium: a general model of neocortical neuronogenesis. J. Neurosci. 16, 6183–6196 (1996).
Cahoy, J.D. et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J. Neurosci. 28, 264–278 (2008).
Polleux, F. & Ghosh, A. The slice overlay assay: a versatile tool to study the influence of extracellular signals on neuronal development. Sci. STKE 136, pl9 (2002).
Molyneaux, B.J., Arlotta, P., Menezes, J.R. & Macklis, J.D. Neuronal subtype specification in the cerebral cortex. Nat. Rev. Neurosci. 8, 427–437 (2007).
Bedogni, F. et al. Tbr1 regulates regional and laminar identity of postmitotic neurons in developing neocortex. Proc. Natl. Acad. Sci. USA 107, 13129–13134 (2010).
Bulfone, A. et al. T-brain-1: a homolog of Brachyury whose expression defines molecularly distinct domains within the cerebral cortex. Neuron 15, 63–78 (1995).
Arlotta, P. et al. Neuronal subtype–specific genes that control corticospinal motor neuron development in vivo. Neuron 45, 207–221 (2005).
Nieto, M. et al. Expression of Cux-1 and Cux-2 in the subventricular zone and upper layers II–IV of the cerebral cortex. J. Comp. Neurol. 479, 168–180 (2004).
Alcamo, E.A. et al. Satb2 regulates callosal projection neuron identity in the developing cerebral cortex. Neuron 57, 364–377 (2008).
Britanova, O. et al. Satb2 is a postmitotic determinant for upper-layer neuron specification in the neocortex. Neuron 57, 378–392 (2008).
McCormick, D.A. & Prince, D.A. Postnatal development of electrophysiological properties of rat cerebral cortical pyramidal neurones. J. Physiol. (Lond.) 393, 743–762 (1987).
Connors, B.W., Gutnick, M.J. & Prince, D.A. Electrophysiological properties of neocortical neurons in vitro. J. Neurophysiol. 48, 1302–1320 (1982).
Maden, M., Gale, E., Kostetskii, I. & Zile, M. Vitamin A–deficient quail embryos have half a hindbrain and other neural defects. Curr. Biol. 6, 417–426 (1996).
Siegenthaler, J.A. et al. Retinoic acid from the meninges regulates cortical neuron generation. Cell 139, 597–609 (2009).
Vallier, L. et al. Early cell fate decisions of human embryonic stem cells and mouse epiblast stem cells are controlled by the same signaling pathways. PLoS ONE 4, e6082 (2009).
Livesey, F.J. & Cepko, C.L. Vertebrate neural cell-fate determination: lessons from the retina. Nat. Rev. Neurosci. 2, 109–118 (2001).
Shen, Q. et al. The timing of cortical neurogenesis is encoded within lineages of individual progenitor cells. Nat. Neurosci. 9, 743–751 (2006).
Caviness, V.S. Jr. et al. Cell output, cell cycle duration and neuronal specification: a model of integrated mechanisms of the neocortical proliferative process. Cereb. Cortex 13, 592–598 (2003).
Groc, L., Gustafsson, B. & Hanse, E. AMPA signaling in nascent glutamatergic synapses: there and not there! Trends Neurosci. 29, 132–139 (2006).
Bystron, I., Blakemore, C. & Rakic, P. Development of the human cerebral cortex: Boulder Committee revisited. Nat. Rev. Neurosci. 9, 110–122 (2008).
Rashid, S.T. et al. Modeling inherited metabolic disorders of the liver using human induced pluripotent stem cells. J. Clin. Invest. 120, 3127–3136 (2010).
Vallier, L. et al. Signaling pathways controlling pluripotency and early cell fate decisions of human induced pluripotent stem cells. Stem Cells 27, 2655–2666 (2009).
Acknowledgements
We thank L. Vallier (Cambridge) and Y. Takashima for kindly providing human iPS cell lines and J. Nichols (Cambridge Centre for Stem Cell Research, Cambridge) for providing the Edi2 human ES cell line. We also thank the members of the Livesey laboratory for their contributions, comments and input to this research. Y.S. was supported by a Biotechnology and Biological Sciences Research Council Dorothy Hodgkin Studentship. P.K. was supported by the University of Cambridge/Wellcome Trust PhD Programme in Developmental Biology. This research benefits from core support to the Gurdon Institute from the Wellcome Trust and Cancer Research UK and grants to F.J.L. from the Wellcome Trust and Alzheimer's Research UK.
Author information
Authors and Affiliations
Contributions
Y.S., P.K., H.P.C.R. and F.J.L. designed the study. Y.S., P.K. and J.S. carried out the experiments. Y.S., P.K., H.P.C.R. and F.J.L. analyzed the data. Y.S., P.K., H.P.C.R. and F.J.L. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 (PDF 0 kb)
Supplementary Movie
Interkinetic nuclear migration, apical and basal mitoses in cortical rosettes. Time-lapse movie of the hESC-derived cortical rosette shown in Figure 2. In the initial frames the blue arrow indicates the apical progenitor and the yellow arrowhead indicates the basal progenitor shown in Figure 2. Frames were collected at 15 minute intervals. (MOV 1432 kb)
Rights and permissions
About this article
Cite this article
Shi, Y., Kirwan, P., Smith, J. et al. Human cerebral cortex development from pluripotent stem cells to functional excitatory synapses. Nat Neurosci 15, 477–486 (2012). https://doi.org/10.1038/nn.3041
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3041
This article is cited by
-
An epigenetic barrier sets the timing of human neuronal maturation
Nature (2024)
-
Induced Pluripotent Stem Cells as a Possible Approach for Exploring the Pathophysiology of Polycystic Ovary Syndrome (PCOS)
Stem Cell Reviews and Reports (2024)
-
Morphological and transcriptomic analyses of stem cell-derived cortical neurons reveal mechanisms underlying synaptic dysfunction in schizophrenia
Genome Medicine (2023)
-
Potential use of iPSCs for disease modeling, drug screening, and cell-based therapy for Alzheimer’s disease
Cellular & Molecular Biology Letters (2023)
-
Directional induction of neural stem cells, a new therapy for neurodegenerative diseases and ischemic stroke
Cell Death Discovery (2023)