Abstract
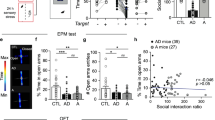
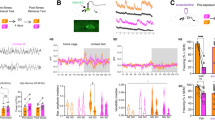
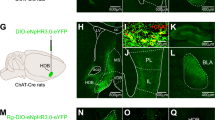
Generalized anxiety is thought to result, in part, from impairments in contingency awareness during conditioning to cues that predict aversive or fearful outcomes. Dopamine neurons of the ventral midbrain exhibit heterogeneous responses to aversive stimuli that are thought to provide a critical modulatory signal to facilitate orientation to environmental changes and assignment of motivational value to unexpected events. Here we describe a mouse model in which activation of dopamine neurons in response to an aversive stimulus is attenuated by conditional genetic inactivation of functional NMDA receptors on dopamine neurons. We discovered that altering the magnitude of excitatory responses by dopamine neurons in response to an aversive stimulus was associated with impaired conditioning to a cue that predicts an aversive outcome. Impaired conditioning by these mice was associated with the development of a persistent, generalized anxiety-like phenotype. These data are consistent with a role for dopamine in facilitating contingency awareness that is critical for the prevention of generalized anxiety.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Wise, R.A. Dopamine, learning and motivation. Nat. Rev. Neurosci. 5, 483–494 (2004).
Mantz, J., Thierry, A.M. & Glowinski, J. Effect of noxious tail pinch on the discharge rate of mesocortical and mesolimbic dopamine neurons: selective activation of the mesocortical system. Brain Res. 476, 377–381 (1989).
Ungless, M.A., Magill, P.J. & Bolam, J.P. Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science 303, 2040–2042 (2004).
Brischoux, F., Chakraborty, S., Brierley, D.I. & Ungless, M.A. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc. Natl. Acad. Sci. USA 106, 4894–4899 (2009).
Chiodo, L.A., Caggiula, A.R., Antelman, S.M. & Lineberry, C.G. Reciprocal influences of activating and immobilizing stimuli on the activity of nigrostriatal dopamine neurons. Brain Res. 176, 385–390 (1979).
Joshua, M., Adler, A., Mitelman, R., Vaadia, E. & Bergman, H. Midbrain dopaminergic neurons and striatal cholinergic interneurons encode the difference between reward and aversive events at different epochs of probabilistic classical conditioning trials. J. Neurosci. 28, 11673–11684 (2008).
Matsumoto, M. & Hikosaka, O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature 459, 837–841 (2009).
Schultz, W. & Romo, R. Responses of nigrostriatal dopamine neurons to high-intensity somatosensory stimulation in the anesthetized monkey. J. Neurophysiol. 57, 201–217 (1987).
Bromberg-Martin, E.S., Matsumoto, M. & Hikosaka, O. Dopamine in motivational control: rewarding, aversive and alerting. Neuron 68, 815–834 (2010).
Lovibond, P.F. & Shanks, D.R. The role of awareness in Pavlovian conditioning: empirical evidence and theoretical implications. J. Exp. Psychol. Anim. Behav. Process. 28, 3–26 (2002).
Grillon, C. Associative learning deficits increase symptoms of anxiety in humans. Biol. Psychiatry 51, 851–858 (2002).
Pezze, M.A. & Feldon, J. Mesolimbic dopaminergic pathways in fear conditioning. Prog. Neurobiol. 74, 301–320 (2004).
Komendantov, A.O., Komendantova, O.G., Johnson, S.W. & Canavier, C.C. A modeling study suggests complementary roles for GABAA and NMDA receptors and the SK channel in regulating the firing pattern in midbrain dopamine neurons. J. Neurophysiol. 91, 346–357 (2004).
Overton, P. & Clark, D. Iontophoretically administered drugs acting at the N-methyl-D-aspartate receptor modulate burst firing in A9 dopamine neurons in the rat. Synapse 10, 131–140 (1992).
Overton, P.G. & Clark, D. Burst firing in midbrain dopaminergic neurons. Brain Res. Brain Res. Rev. 25, 312–334 (1997).
Zweifel, L.S. et al. Disruption of NMDAR-dependent burst firing by dopamine neurons provides selective assessment of phasic dopamine-dependent behavior. Proc. Natl. Acad. Sci. USA 106, 7281–7288 (2009).
Zweifel, L.S., Argilli, E., Bonci, A. & Palmiter, R.D. Role of NMDA receptors in dopamine neurons for plasticity and addictive behaviors. Neuron 59, 486–496 (2008).
Robinson, S., Smith, D.M., Mizumori, S.J. & Palmiter, R.D. Firing properties of dopamine neurons in freely moving dopamine-deficient mice: effects of dopamine receptor activation and anesthesia. Proc. Natl. Acad. Sci. USA 101, 13329–13334 (2004).
Chiodo, L.A., Antelman, S.M., Caggiula, A.R. & Lineberry, C.G. Sensory stimuli alter the discharge rate of dopamine (DA) neurons: evidence for two functional types of DA cells in the substantia nigra. Brain Res. 189, 544–549 (1980).
Margolis, E.B., Lock, H., Hjelmstad, G.O. & Fields, H.L. The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons? J. Physiol. (Lond.) 577, 907–924 (2006).
Luo, A.H., Georges, F.E. & Aston-Jones, G.S. Novel neurons in ventral tegmental area fire selectively during the active phase of the diurnal cycle. Eur. J. Neurosci. 27, 408–422 (2008).
Davis, M., Falls, W.A., Campeau, S. & Kim, M. Fear-potentiated startle: a neural and pharmacological analysis. Behav. Brain Res. 58, 175–198 (1993).
Fadok, J.P., Dickerson, T.M. & Palmiter, R.D. Dopamine is necessary for cue-dependent fear conditioning. J. Neurosci. 29, 11089–11097 (2009).
Richardson, R. Shock sensitization of startle: learned or unlearned fear? Behav. Brain Res. 110, 109–117 (2000).
Grillon, C. Startle reactivity and anxiety disorders: aversive conditioning, context and neurobiology. Biol. Psychiatry 52, 958–975 (2002).
Koch, M. & Schnitzler, H.U. The acoustic startle response in rats—circuits mediating evocation, inhibition and potentiation. Behav. Brain Res. 89, 35–49 (1997).
Hunter, A.J., Nolan, P.M. & Brown, S.D. Towards new models of disease and physiology in the neurosciences: the role of induced and naturally occurring mutations. Hum. Mol. Genet. 9, 893–900 (2000).
Ralph, R.J. et al. The dopamine D2, but not D3 or D4, receptor subtype is essential for the disruption of prepulse inhibition produced by amphetamine in mice. J. Neurosci. 19, 4627–4633 (1999).
Grillon, C. et al. Cortisol and DHEA-S are associated with startle potentiation during aversive conditioning in humans. Psychopharmacology (Berl.) 186, 434–441 (2006).
Jovanovic, T. et al. Fear potentiation is associated with hypothalamic-pituitary-adrenal axis function in PTSD. Psychoneuroendocrinology 35, 846–857 (2010).
Sánchez, M.M. et al. Alterations in diurnal cortisol rhythm and acoustic startle response in nonhuman primates with adverse rearing. Biol. Psychiatry 57, 373–381 (2005).
Flood, D.G., Zuvich, E., Marino, M.J. & Gasior, M. The effects of d-amphetamine, methylphenidate, sydnocarb and caffeine on prepulse inhibition of the startle reflex in DBA/2 mice. Psychopharmacology (Berl.) 211, 325–336 (2010).
Ungless, M.A., Whistler, J.L., Malenka, R.C. & Bonci, A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature 411, 583–587 (2001).
Lammel, S. et al. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron 57, 760–773 (2008).
LeDoux, J.E. Emotion circuits in the brain. Annu. Rev. Neurosci. 23, 155–184 (2000).
Swerdlow, N.R. et al. Toward understanding the biology of a complex phenotype: rat strain and substrain differences in the sensorimotor gating-disruptive effects of dopamine agonists. J. Neurosci. 20, 4325–4336 (2000).
Paxinos, G. & Franklin, K.B.J. The Mouse Brain in Stereotaxic Coordinates (Academic Press, San Diego, California, 2001)
Acknowledgements
We thank A.D. Guler and members of the Palmiter laboratory for thoughtful discussion of this manuscript. We thank G. Froelich, V. Wall and M.J. Kim for technical support. This work was supported in part by US National Institutes of Health grants 2T32 GM007270 (to J.P.F.), 4 R25 GM 058501-05 (to T.M.K.D.) and 1 R01 MH58755 (to S.J.Y.M.).
Author information
Authors and Affiliations
Contributions
L.S.Z. and J.P.F. designed the experiments. L.S.Z. performed in vivo recordings with assistance from G.L.J. and S.J.Y.M. L.S.Z. and J.P.F. performed behavioral experiments with assistance from M.G.G. and T.M.K.D. E.A. performed slice physiology with support from A.B. R.D.P. constructed the AAV1-fs-HA-NR1 viral vector. J.M.A. purified AAV1-fs-HA-NR1. The manuscript was written by L.S.Z. with assistance from J.P.F. and R.D.P.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4 (PDF 2406 kb)
Rights and permissions
About this article
Cite this article
Zweifel, L., Fadok, J., Argilli, E. et al. Activation of dopamine neurons is critical for aversive conditioning and prevention of generalized anxiety. Nat Neurosci 14, 620–626 (2011). https://doi.org/10.1038/nn.2808
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2808
This article is cited by
-
An ACC–VTA–ACC positive-feedback loop mediates the persistence of neuropathic pain and emotional consequences
Nature Neuroscience (2024)
-
D1 receptor-expressing neurons in ventral tegmental area alleviate mouse anxiety-like behaviors via glutamatergic projection to lateral septum
Molecular Psychiatry (2023)
-
Mood and behavior regulation: interaction of lithium and dopaminergic system
Naunyn-Schmiedeberg's Archives of Pharmacology (2023)
-
Collateral projections from the ventral tegmental area/substantia nigra pars compacta to the nucleus accumbens and insular cortex in the rat
Anatomical Science International (2023)
-
Intermittent Restraint Stress and Recovery on GPCR Expression in Brain/Gut Axis of Mice: Activation of Adrenergic, Dopamine, Serotonin and Melatonin Receptors During Stress and Ease Response
Proceedings of the Zoological Society (2023)