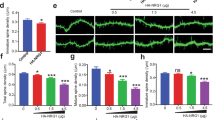
Abstract
Synaptic spines are dynamic structures that regulate neuronal responsiveness and plasticity. We examined the role of the schizophrenia risk factor DISC1 in the maintenance of spine morphology and function. We found that DISC1 anchored Kalirin-7 (Kal-7), regulating access of Kal-7 to Rac1 and controlling the duration and intensity of Rac1 activation in response to NMDA receptor activation in both cortical cultures and rat brain in vivo. These results explain why Rac1 and its activator (Kal-7) serve as important mediators of spine enlargement and why constitutive Rac1 activation decreases spine size. This mechanism likely underlies disturbances in glutamatergic neurotransmission that have been frequently reported in schizophrenia that can lead to alteration of dendritic spines with consequential major pathological changes in brain function. Furthermore, the concept of a signalosome involving disease-associated factors, such as DISC1 and glutamate, may well contribute to the multifactorial and polygenetic characteristics of schizophrenia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Goff, D.C. & Coyle, J.T. The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am. J. Psychiatry 158, 1367–1377 (2001).
Moghaddam, B. Bringing order to the glutamate chaos in schizophrenia. Neuron 40, 881–884 (2003).
McCullumsmith, R.E., Clinton, S.M. & Meador-Woodruff, J.H. Schizophrenia as a disorder of neuroplasticity. Int. Rev. Neurobiol. 59, 19–45 (2004).
Glantz, L.A. & Lewis, D.A. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch. Gen. Psychiatry 57, 65–73 (2000).
Garey, L.J. et al. Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. J. Neurol. Neurosurg. Psychiatry 65, 446–453 (1998).
Maletic-Savatic, M., Malinow, R. & Svoboda, K. Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science 283, 1923–1927 (1999).
Matsuzaki, M., Honkura, N., Ellis-Davies, G.C. & Kasai, H. Structural basis of long-term potentiation in single dendritic spines. Nature 429, 761–766 (2004).
Steen, R.G., Hamer, R.M. & Lieberman, J.A. Measurement of brain metabolites by 1H magnetic resonance spectroscopy in patients with schizophrenia: a systematic review and meta-analysis. Neuropsychopharmacology 30, 1949–1962 (2005).
Pilowsky, L.S. et al. First in vivo evidence of an NMDA receptor deficit in medication-free schizophrenic patients. Mol. Psychiatry 11, 118–119 (2006).
Mirnics, K., Middleton, F.A., Lewis, D.A. & Levitt, P. Analysis of complex brain disorders with gene expression microarrays: schizophrenia as a disease of the synapse. Trends Neurosci. 24, 479–486 (2001).
Hill, J.J., Hashimoto, T. & Lewis, D.A. Molecular mechanisms contributing to dendritic spine alterations in the prefrontal cortex of subjects with schizophrenia. Mol. Psychiatry 11, 557–566 (2006).
Fischer, M., Kaech, S., Knutti, D. & Matus, A. Rapid actin-based plasticity in dendritic spines. Neuron 20, 847–854 (1998).
Carlisle, H.J. & Kennedy, M.B. Spine architecture and synaptic plasticity. Trends Neurosci. 28, 182–187 (2005).
Harrison, P.J. & West, V.A. Six degrees of separation: on the prior probability that schizophrenia susceptibility genes converge on synapses, glutamate and NMDA receptors. Mol. Psychiatry 11, 981–983 (2006).
Hashimoto, R., Tankou, S., Takeda, M. & Sawa, A. Postsynaptic density: a key convergent site for schizophrenia susceptibility factors and possible target for drug development. Drugs Today (Barc) 43, 645–654 (2007).
Sheng, M. & Hoogenraad, C.C. The postsynaptic architecture of excitatory synapses: a more quantitative view. Annu. Rev. Biochem. 76, 823–847 (2007).
Blanpied, T.A., Kerr, J.M. & Ehlers, M.D. Structural plasticity with preserved topology in the postsynaptic protein network. Proc. Natl. Acad. Sci. USA 105, 12587–12592 (2008).
St. Clair, D. et al. Association within a family of a balanced autosomal translocation with major mental illness. Lancet 336, 13–16 (1990).
Callicott, J.H. et al. Variation in DISC1 affects hippocampal structure and function and increases risk for schizophrenia. Proc. Natl. Acad. Sci. USA 102, 8627–8632 (2005).
Cannon, T.D. et al. Association of DISCC1/TRAX haplotypes with schizophrenia, reduced prefrontal gray matter, and impaired short- and long-term memory. Arch. Gen. Psychiatry 62, 1205–1213 (2005).
Ishizuka, K., Paek, M., Kamiya, A. & Sawa, A. A review of Disrupted-In-Schizophrenia-1 (DISC1): neurodevelopment, cognition, and mental conditions. Biol. Psychiatry 59, 1189–1197 (2006).
Chubb, J.E., Bradshaw, N.J., Soares, D.C., Porteous, D.J. & Millar, J.K. The DISC locus in psychiatric illness. Mol. Psychiatry 13, 36–64 (2008).
Kirkpatrick, B. et al. DISC1 immunoreactivity at the light and ultrastructural level in the human neocortex. J. Comp. Neurol. 497, 436–450 (2006).
Kvajo, M. et al. A mutation in mouse Disc1 that models a schizophrenia risk allele leads to specific alterations in neuronal architecture and cognition. Proc. Natl. Acad. Sci. USA 105, 7076–7081 (2008).
Kamiya, A. et al. A schizophrenia-associated mutation of DISC1 perturbs cerebral cortex development. Nat. Cell Biol. 7, 1167–1178 (2005).
Shi, S.H. et al. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science 284, 1811–1816 (1999).
Xie, Z. et al. Kalirin-7 controls activity-dependent structural and functional plasticity of dendritic spines. Neuron 56, 640–656 (2007).
Ma, X.M., Wang, Y., Ferraro, F., Mains, R.E. & Eipper, B.A. Kalirin-7 is an essential component of both shaft and spine excitatory synapses in hippocampal interneurons. J. Neurosci. 28, 711–724 (2008).
Cahill, M. et al. Kalirin regulates cortical spine morphogenesis and disease-related behavioral phenotypes. Proc. Natl. Acad. Sci. USA 106, 13058–13063 (2009).
Saneyoshi, T. et al. Activity-dependent synaptogenesis: regulation by a CaM-kinase kinase/CaM-kinase I/betaPIX signaling complex. Neuron 57, 94–107 (2008).
Tolias, K.F. et al. The Rac1-GEF Tiam1 couples the NMDA receptor to the activity-dependent development of dendritic arbors and spines. Neuron 45, 525–538 (2005).
Bolger, G.B. et al. Scanning peptide array analyses identify overlapping binding sites for the signalling scaffold proteins, beta-arrestin and RACK1, in cAMP-specific phosphodiesterase PDE4D5. Biochem. J. 398, 23–36 (2006).
Millar, J.K. et al. Drosoph. Inf. Serv.C1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science 310, 1187–1191 (2005).
Murdoch, H. et al. Isoform-selective susceptibility of DISC1/phosphodiesterase-4 complexes to dissociation by elevated intracellular cAMP levels. J. Neurosci. 27, 9513–9524 (2007).
Taya, S. et al. DISC1 regulates the transport of the NUDEL/LIS1/14–3-3epsilon complex through kinesin-1. J. Neurosci. 27, 15–26 (2007).
Steward, O. & Worley, P.F. Selective targeting of newly synthesized Arc mRNA to active synapses requires NMDA receptor activation. Neuron 30, 227–240 (2001).
Hsueh, Y.P., Kim, E. & Sheng, M. Disulfide-linked head-to-head multimerization in the mechanism of ion channel clustering by PSD-95. Neuron 18, 803–814 (1997).
Djinovic-Carugo, K., Gautel, M., Ylanne, J. & Young, P. The spectrin repeat: a structural platform for cytoskeletal protein assemblies. FEBS Lett. 513, 119–123 (2002).
Rabiner, C.A., Mains, R.E. & Eipper, B.A. Kalirin: a dual Rho guanine nucleotide exchange factor that is so much more than the sum of its many parts. Neuroscientist 11, 148–160 (2005).
Luo, L. et al. Differential effects of the Rac GTPase on Purkinje cell axons and dendritic trunks and spines. Nature 379, 837–840 (1996).
Tashiro, A., Minden, A. & Yuste, R. Regulation of dendritic spine morphology by the rho family of small GTPases: antagonistic roles of Rac and Rho. Cereb. Cortex 10, 927–938 (2000).
Camargo, L.M. et al. Disrupted in Schizophrenia 1 interactome: evidence for the close connectivity of risk genes and a potential synaptic basis for schizophrenia. Mol. Psychiatry 12, 74–86 (2007).
Ishizuka, K. et al. Evidence that many of the DISC1 isoforms in C57BL/6J mice are also expressed in 129S6/SvEv mice. Mol. Psychiatry 12, 897–899 (2007).
Jaaro-Peled, H. et al. Neurodevelopmental mechanisms of schizophrenia: understanding disturbed postnatal brain maturation through Neuregulin-1 and DISC1. Trends Neurosci. 32, 485–495 (2009).
McGlashan, T.H. & Hoffman, R.E. Schizophrenia as a disorder of developmentally reduced synaptic connectivity. Arch. Gen. Psychiatry 57, 637–648 (2000).
Schurov, I.L., Handford, E.J., Brandon, N.J. & Whiting, P.J. Expression of disrupted in schizophrenia 1 (DISC1) protein in the adult and developing mouse brain indicates its role in neurodevelopment. Mol. Psychiatry 9, 1100–1110 (2004).
Holtmaat, A.J. et al. Transient and persistent dendritic spines in the neocortex in vivo. Neuron 45, 279–291 (2005).
McGlashan, T.H. & Johannessen, J.O. Early detection and intervention with schizophrenia: rationale. Schizophr. Bull. 22, 201–222 (1996).
Koike, H., Arguello, P.A., Kvajo, M., Karayiorgou, M. & Gogos, J.A. Disc1 is mutated in the 129S6/SvEv strain and modulates working memory in mice. Proc. Natl. Acad. Sci. USA 103, 3693–3697 (2006).
Cai, X., Gu, Z., Zhong, P., Ren, Y. & Yan, Z. Serotonin 5-HT1A receptors regulate AMPA receptor channels through inhibiting Ca2+/calmodulin-dependent kinase II in prefrontal cortical pyramidal neurons. J. Biol. Chem. 277, 36553–36562.
Acknowledgements
We thank Y. Lema and P. Talalay for help with manuscript preparation, A. Gruber, P. O'Donnell, P.F. Worley, R.L. Huganir, J.D. Rothstein, N. Shahani, H. Ujike, S. Kuroda, H. Bito and M. Nuriya for scientific discussions and J. Gogos, R.A. Cerione, H. Cline and A. Jeromin for providing us with reagents. This work was supported by grants from the US National Institutes of Health (MH-084018, MH-069853 and MH-088753 to A.S., MH-071316 to P.P., and MH-084233 and NS-048911 to Z.Y.), as well as by grants from Stanley (A.S.), Cure Huntington's Disease Initiative (A.S.), HighQ (A.S.), S & R Foundation (A.S.), RUSK (A.S.), National Alliance for Research on Schizophrenia and Depression (A.S., A.H.-T., A.K. and P.P.), National Alliance for Autism Research (P.P.), Uehara (A.H.-T. and M.T.), Medical Research Council (G0600765 to M.D.H.) and the European Union (LSHB-CT-2006-037189 to M.D.H.).
Author information
Authors and Affiliations
Contributions
A.H.-T., M.T., N.G., S.S., H.M., A.J.D. and T.T. conducted the experiments. Y.M., A.J.S., K.I., D.P.S. and Z.X. provided assistance for the experiments. J.M.B., M.D.H., T.T., N.J.B., A.K., Z.Y. and P.P. contributed to experimental design. A.H.-T., M.D.H., N.J.B. and A.S. wrote the manuscript. A.S. led the overall experimental design of the entire project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–18 (PDF 3226 kb)
Rights and permissions
About this article
Cite this article
Hayashi-Takagi, A., Takaki, M., Graziane, N. et al. Disrupted-in-Schizophrenia 1 (DISC1) regulates spines of the glutamate synapse via Rac1. Nat Neurosci 13, 327–332 (2010). https://doi.org/10.1038/nn.2487
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2487
This article is cited by
-
Decreased CNNM2 expression in prefrontal cortex affects sensorimotor gating function, cognition, dendritic spine morphogenesis and risk of schizophrenia
Neuropsychopharmacology (2024)
-
Metabolic enzyme LDHA activates Rac1 GTPase as a noncanonical mechanism to promote cancer
Nature Metabolism (2022)
-
Pathological oligodendrocyte precursor cells revealed in human schizophrenic brains and trigger schizophrenia-like behaviors and synaptic defects in genetic animal model
Molecular Psychiatry (2022)
-
Kalirin as a Novel Treatment Target for Cognitive Dysfunction in Schizophrenia
CNS Drugs (2022)
-
Impairment of early neuronal maturation in anti-NMDA-receptor encephalitis
Psychopharmacology (2022)