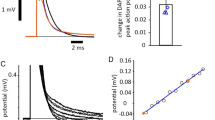
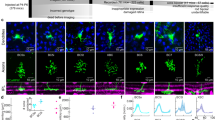
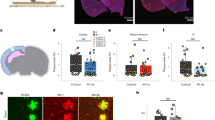
Abstract
The perception of migraine headache, which is mediated by nociceptive signals transmitted from the cranial dura mater to the brain, is uniquely exacerbated by exposure to light. We found that exacerbation of migraine headache by light is prevalent among blind individuals who maintain non–image-forming photoregulation in the face of massive rod/cone degeneration. Using single-unit recording and neural tract tracing in the rat, we identified dura-sensitive neurons in the posterior thalamus whose activity was distinctly modulated by light and whose axons projected extensively across layers I–V of somatosensory, visual and associative cortices. The cell bodies and dendrites of such dura/light-sensitive neurons were apposed by axons originating from retinal ganglion cells (RGCs), predominantly from intrinsically photosensitive RGCs, the principle conduit of non–image-forming photoregulation. We propose that photoregulation of migraine headache is exerted by a non–image-forming retinal pathway that modulates the activity of dura-sensitive thalamocortical neurons.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Headache Classification Subcommittee of the International Headache Society. The international classification of headache disorders: second edition. Cephalalgia 24, 9–160 (2004).
Selby, G. & Lance, J.W. Observations on 500 cases of migraine and allied vascular headache. J. Neurol. Neurosurg. Psychiatry 23, 23–32 (1960).
Markowitz, S., Saito, K. & Moskowitz, M.A. Neurogenically mediated plasma extravasation in dura mater: effect of ergot alkaloids. A possible mechanism of action in vascular headache. Cephalalgia 8, 83–91 (1988).
Penfield, W. & McNaughton, F. Dural headache and innervation of the dura mater. Arch. Neurol. Psychiatry 44, 43–75 (1940).
Burstein, R., Yamamura, H., Malick, A. & Strassman, A.M. Chemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brain stem trigeminal neurons. J. Neurophysiol. 79, 964–982 (1998).
Strassman, A.M., Raymond, S.A. & Burstein, R. Sensitization of meningeal sensory neurons and the origin of headaches. Nature 384, 560–564 (1996).
Burstein, R., Yarnitsky, D., Goor-Aryeh, I., Ransil, B.J. & Bajwa, Z.H. An association between migraine and cutaneous allodynia. Ann. Neurol. 47, 614–624 (2000).
Burstein, R., Cutrer, F.M. & Yarnitsky, D. The development of cutaneous allodynia during a migraine attack: clinical evidence for the sequential recruitment of spinal and supraspinal nociceptive neurons in migraine. Brain 123, 1703–1709 (2000).
Kawasaki, A. & Purvin, V.A. Photophobia as the presenting visual symptom of chiasmal compression. J. Neuroophthalmol. 22, 3–8 (2002).
Liveing, E. On Megrim, Sick Headache (Arts & Boeve Publishers, Nijmegen, The Netherlands, 1873).
Lebensohn, J.E. Photophobia: mechanism and implications. Am. J. Ophthalmol. 34, 1294–1300 (1951).
Aurora, S.K., Cao, Y., Bowyer, S.M. & Welch, K.M. The occipital cortex is hyperexcitable in migraine: experimental evidence. Headache 39, 469–476 (1999).
Lamonte, M., Silberstein, S.D. & Marcelis, J.F. Headache associated with aseptic meningitis. Headache 35, 520–526 (1995).
Welty, T.E. & Horner, T.G. Pathophysiology and treatment of subarachnoid hemorrhage. Clin. Pharm. 9, 35–39 (1990).
Lowenfeld, I. The Dazzling Syndrome (Wane State University Press, Detroit, Michigan, USA, 1993).
Miller, N.R. Photophobia. in Walsh and Hoyt's Clinical Neuro-ophthlmology (ed. N.R. Miller) 1099–1106 (Williams & Wilkins, Baltimore, 1985).
Lucas, R.J., Douglas, R.H. & Foster, R.G. Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nat. Neurosci. 4, 621–626 (2001).
Klein, D.C. & Weller, J.L. Rapid light-induced decrease in pineal serotonin N-acetyltransferase activity. Science 177, 532–533 (1972).
Freedman, M.S. et al. Regulation of mammalian circadian behavior by non-rod, non-cone, ocular photoreceptors. Science 284, 502–504 (1999).
Hattar, S. et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature 424, 76–81 (2003).
Lucas, R.J., Freedman, M.S., Munoz, M., Garcia-Fernandez, J.M. & Foster, R.G. Regulation of the mammalian pineal by non-rod, non-cone, ocular photoreceptors. Science 284, 505–507 (1999).
Gooley, J.J., Lu, J., Fischer, D. & Saper, C.B. A broad role for melanopsin in nonvisual photoreception. J. Neurosci. 23, 7093–7106 (2003).
Güler, A.D. et al. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature 453, 102–105 (2008).
Hattar, S. et al. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J. Comp. Neurol. 497, 326–349 (2006).
Hannibal, J. & Fahrenkrug, J. Target areas innervated by PACAP-immunoreactive retinal ganglion cells. Cell Tissue Res. 316, 99–113 (2004).
Provencio, I., Jiang, G., De Grip, W.J., Hayes, W.P. & Rollag, M.D. Melanopsin: An opsin in melanophores, brain and eye. Proc. Natl. Acad. Sci. USA 95, 340–345 (1998).
Provencio, I. et al. A novel human opsin in the inner retina. J. Neurosci. 20, 600–605 (2000).
Berson, D.M., Dunn, F.A. & Takao, M. Phototransduction by retinal ganglion cells that set the circadian clock. Science 295, 1070–1073 (2002).
Hattar, S., Liao, H.W., Takao, M., Berson, D.M. & Yau, K.W. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science 295, 1065–1070 (2002).
Panda, S. et al. Melanopsin is required for non-image-forming photic responses in blind mice. Science 301, 525–527 (2003).
Malick, A., Jakubowski, M., Elmquist, J.K., Saper, C.B. & Burstein, R. A neurohistochemical blueprint for pain-induced loss of appetite. Proc. Natl. Acad. Sci. USA 98, 9930–9935 (2001).
Zagami, A.S. & Lambert, G.A. Stimulation of cranial vessels excites nociceptive neurones in several thalamic nuclei of the cat. Exp. Brain Res. 81, 552–566 (1990).
Davis, K.D. & Dostrovsky, J.O. Properties of feline thalamic neurons activated by stimulation of the middle meningeal artery and sagittal sinus. Brain Res. 454, 89–100 (1988).
Dacey, D.M. et al. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature 433, 749–754 (2005).
Wong, K.Y., Dunn, F.A., Graham, D.M. & Berson, D.M. Synaptic influences on rat ganglion-cell photoreceptors. J. Physiol. (Lond.) 582, 279–296 (2007).
Hannibal, J. et al. Melanopsin is expressed in PACAP-containing retinal ganglion cells of the human retinohypothalamic tract. Invest. Ophthalmol. Vis. Sci. 45, 4202–4209 (2004).
Berson, D.M. Phototransduction in ganglion-cell photoreceptors. Pflugers Arch. 454, 849–855 (2007).
Tu, D.C. et al. Physiologic diversity and development of intrinsically photosensitive retinal ganglion cells. Neuron 48, 987–999 (2005).
Herkenham, M. Laminar organization of thalamic projections to the rat neocortex. Science 207, 532–535 (1980).
Ingvar, M. & Hsieh, J.-C. The image of pain. in Textbook of Pain (eds P.D. Wall & R. Melzack) 215–233 (Churchill Livingston, London, 1999).
Valenstein, E. et al. Retrosplenial amnesia. Brain 110, 1631–1646 (1987).
Donchin, O., Gribova, A., Steinberg, O., Bergman, H. & Vaadia, E. Primary motor cortex is involved in bimanual coordination. Nature 395, 274–278 (1998).
Mountcastle, V.B., Lynch, J.C., Georgopoulos, A., Sakata, H. & Acuna, C. Posterior parietal association cortex of the monkey: command functions for operations within extrapersonal space. J. Neurophysiol. 38, 871–908 (1975).
Lowel, S. & Singer, W. Selection of intrinsic horizontal connections in the visual cortex by correlated neuronal activity. Science 255, 209–212 (1992).
Livingstone, M.S. & Hubel, D.H. Anatomy and physiology of a color system in the primate visual cortex. J. Neurosci. 4, 309–356 (1984).
Paxinos, G. & Watson, C. The Rat Brain in Stereotaxic Coordinates (Academic Press, Orlando, Florida, USA, 1998).
Burstein, R. & Jakubowski, M. Analgesic triptan action in an animal model of intracranial pain: a race against the development of central sensitization. Ann. Neurol. 55, 27–36 (2004).
Yamamura, H., Malick, A., Chamberlin, N.L. & Burstein, R. Cardiovascular and neuronal responses to head stimulation reflect central sensitization and cutaneous allodynia in a rat model of migraine. J. Neurophysiol. 81, 479–493 (1999).
Pinault, D. A novel single-cell staining procedure performed in vivo under electrophysiological control: Morpho-functional features of juxtacellularly labeled thalamic cells and other central neurons with biocytin or Neurobiotin. J. Neurosci. Methods 65, 113–136 (1996).
Gauriau, C. & Bernard, J.F. Posterior triangular thalamic neurons convey nociceptive messages to the secondary somatosensory and insular cortices in the rat. J. Neurosci. 24, 752–761 (2004).
Acknowledgements
We thank D. Friedman, A. Recober, M. Carmen-Wilson and A. Mauskop for sharing their blind migraine patients, and L. Villanueva and J.-F. Bernard for teaching us the juxtacellular labeling technique. This research was supported by US National Institutes of Health grants NS-051484 and NS-035611. K.D. was supported in part by an unrestricted grant from Research to Prevent Blindness.
Author information
Authors and Affiliations
Contributions
R.B., M.J. and R.N. designed the study. R.N., V.K., J.J.G. and R.B. conducted the various experiments. M.J., R.N., C.B.S. and K.D. contributed to data analysis and presentation. R.B. and M.J. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 and Supplementary Table 1 (PDF 26609 kb)
Rights and permissions
About this article
Cite this article
Noseda, R., Kainz, V., Jakubowski, M. et al. A neural mechanism for exacerbation of headache by light. Nat Neurosci 13, 239–245 (2010). https://doi.org/10.1038/nn.2475
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2475
This article is cited by
-
Mapping the aberrant brain functional connectivity in new daily persistent headache: a resting-state functional magnetic resonance imaging study
The Journal of Headache and Pain (2023)
-
Neuropeptide Y in the medial habenula alleviates migraine-like behaviors through the Y1 receptor
The Journal of Headache and Pain (2023)
-
Galcanezumab effects on incidence of headache after occurrence of triggers, premonitory symptoms, and aura in responders, non-responders, super-responders, and super non-responders
The Journal of Headache and Pain (2023)
-
Brain structure and cortical activity changes of new daily persistent headache: multimodal evidence from MEG/sMRI
The Journal of Headache and Pain (2023)
-
Calcitonin gene-related peptide causes migraine aura
The Journal of Headache and Pain (2023)