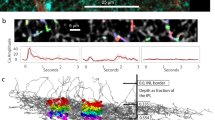
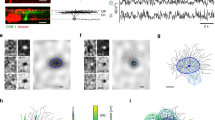
Abstract
In the visual system of the blowfly Calliphora vicina, neurons of the vertical system (VS cells) integrate wide-field motion information from a retinotopic array of local motion detectors. In vivo calcium imaging reveals two distinct and separate receptive fields in these cells: a narrow dendritic receptive field corresponding to feedforward input from the local motion detectors and a broad axon terminal receptive field that additionally incorporates input from neighboring cells mediated by lateral axo-axonal gap junctions. We show that the axon terminal responses are linear interpolations of the dendritic responses, resulting in a robust population coding of optic flow parameters as predicted by previous modeling studies. Compartmental modeling shows that spatially separating the axonal gap junctions from the conductive load of the dendritic synapses increases the coupling strength of the gap junctions, making this interpolation possible.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Angelucci, A. & Bressloff, P.C. Contribution of feedforward, lateral and feedback connections to the classical receptive field center and extra-classical receptive field surround of primate V1 neurons. Prog. Brain Res. 154, 93–120 (2006).
Finn, I.M., Priebe, N.J. & Ferster, D. The emergence of contrast-invariant orientation tuning in simple cells of cat visual cortex. Neuron 54, 137–152 (2007).
Olsen, S.R., Bhandawat, V. & Wilson, R.I. Excitatory interactions between olfactory processing channels in the Drosophila antennal lobe. Neuron 54, 89–103 (2007).
Shang, Y., Claridge-Chang, A., Sjulson, L., Pypaert, M. & Miesenbock, G. Excitatory local circuits and their implications for olfactory processing in the fly antennal lobe. Cell 128, 601–612 (2007).
MacLeod, K. & Laurent, G. Distinct mechanisms for synchronization and temporal patterning of odor-encoding neural assemblies. Science 274, 976–979 (1996).
Mori, K., Nagao, H. & Yoshihara, Y. The olfactory bulb: coding and processing of odor molecule information. Science 286, 711–715 (1999).
Lei, H., Christensen, T.A. & Hildebrand, J.G. Local inhibition modulates odor-evoked synchronization of glomerulus-specific output neurons. Nat. Neurosci. 5, 557–565 (2002).
Olsen, S.R. & Wilson, R.I. Lateral presynaptic inhibition mediates gain control in an olfactory circuit. Nature 452, 956–960 (2008).
DeVries, S.H. & Baylor, D.A. Synaptic circuitry of the retina and olfactory bulb. Cell 72, Suppl., 139–149 (1993).
Balboa, R.M. & Grzywacz, N.M. The role of early retinal lateral inhibition: more than maximizing luminance information. Vis. Neurosci. 17, 77–89 (2000).
Masland, R.H. The fundamental plan of the retina. Nat. Neurosci. 4, 877–886 (2001).
Wassle, H. Parallel processing in the mammalian retina. Nat. Rev. Neurosci. 5, 747–757 (2004).
Yagi, T. Interaction between the soma and the axon terminal of retinal horizontal cells in Cyprinus carpio. J. Physiol. (Lond.) 375, 121–135 (1986).
Agmon-Snir, H., Carr, C.E. & Rinzel, J. The role of dendrites in auditory coincidence detection. Nature 393, 268–272 (1998).
Koch, C., Poggio, T. & Torre, V. Nonlinear interactions in a dendritic tree: localization, timing, and role in information processing. Proc. Natl. Acad. Sci. USA 80, 2799–2802 (1983).
Archie, K.A. & Mel, B.W. A model for intradendritic computation of binocular disparity. Nat. Neurosci. 3, 54–63 (2000).
Poirazi, P., Brannon, T. & Mel, B.W. Arithmetic of subthreshold synaptic summation in a model CA1 pyramidal cell. Neuron 37, 977–987 (2003).
Polsky, A., Mel, B.W. & Schiller, J. Computational subunits in thin dendrites of pyramidal cells. Nat. Neurosci. 7, 621–627 (2004).
Liu, G. Local structural balance and functional interaction of excitatory and inhibitory synapses in hippocampal dendrites. Nat. Neurosci. 7, 373–379 (2004).
Poirazi, P., Brannon, T. & Mel, B.W. Pyramidal neuron as two-layer neural network. Neuron 37, 989–999 (2003).
Haag, J. & Borst, A. Neural mechanism underlying complex receptive field properties of motion-sensitive interneurons. Nat. Neurosci. 7, 628–634 (2004).
Farrow, K., Borst, A. & Haag, J. Sharing receptive fields with your neighbors: tuning the vertical system cells to wide field motion. J. Neurosci. 25, 3985–3993 (2005).
Borst, A. & Haag, J. Neural networks in the cockpit of the fly. J. Comp. Physiol. [A] 188, 419–437 (2002).
Krapp, H.G., Hengstenberg, B. & Hengstenberg, R. Dendritic structure and receptive-field organization of optic flow processing interneurons in the fly. J. Neurophysiol. 79, 1902–1917 (1998).
Cuntz, H., Haag, J., Forstner, F., Segev, I. & Borst, A. Robust coding of flow-field parameters by axo-axonal gap junctions between fly visual interneurons. Proc. Natl. Acad. Sci. USA 104, 10229–10233 (2007).
Haag, J. & Borst, A. Spatial distribution and characteristics of voltage-gated calcium signals within visual interneurons. J. Neurophysiol. 83, 1039–1051 (2000).
Weber, F., Eichner, H., Cuntz, H. & Borst, A. Eigenanalysis of a neural network for optic flow processing. New J. Phys. 10, 015013 (2008).
Reisenman, C., Haag, J. & Borst, A. Adaptation of response transients in fly motion vision. I: Experiments. Vision Res. 43, 1293–1309 (2003).
Single, S. & Borst, A. Dendritic integration and its role in computing image velocity. Science 281, 1848–1850 (1998).
Borst, A., Egelhaaf, M. & Haag, J. Mechanisms of dendritic integration underlying gain control in fly motion-sensitive interneurons. J. Comput. Neurosci. 2, 5–18 (1995).
van Hateren, J.H. & van der Schaaf, A . Independent component filters of natural images compared with simple cells in primary visual cortex. Proc. Biol. Sci. 265, 359–366 (1998).
Reichardt, W. Evaluation of optical motion information by movement detectors. J. Comp. Physiol. [A] 161, 533–547 (1987).
Borst, A., Reisenman, C. & Haag, J. Adaptation of response transients in fly motion vision. II: Model studies. Vision Res. 43, 1311–1324 (2003).
Pierantoni, R. A look into the cock-pit of the fly. Cell Tissue Res. 171, 101–122 (1976).
Egelhaaf, M. On the neuronal basis of figure-ground discrimination by relative motion in the visual system of the fly. II. Figure-detection cells, a new class of visual interneurones. Biol. Cybern. 52, 195–209 (1985).
Hausen, K. Motion sensitive interneurons in the optomotor system of the fly. I. The horizontal cells: Structure and signals. Biol. Cybern. 45, 143–156 (1982).
Eckert, H. & Dvorak, D.R. The centrifugal horizontal cells in the lobula plate of the blowfly Phaenicia sericata. J. Insect Physiol. 29, 547–560 (1983).
Hausen, K., Wolburg-Buchholz, K. & Ribi, W.A. The synaptic organization of visual interneurons in the lobula complex of flies. Cell Tissue Res. 208, 371–387 (1980).
Wertz, A., Borst, A. & Haag, J. Nonlinear integration of binocular optic flow by DNOVS2, a descending neuron of the fly. J. Neurosci. 28, 3131–3140 (2008).
Llinas, R., Baker, R. & Sotelo, C. Electrotonic coupling between neurons in cat inferior olive. J. Neurophysiol. 37, 560–571 (1974).
Lang, E.J., Sugihara, I. & Llinas, R. GABAergic modulation of complex spike activity by the cerebellar nucleoolivary pathway in rat. J. Neurophysiol. 76, 255–275 (1996).
Spira, M.E. & Bennett, M.V. Synaptic control of electrotonic coupling between neurons. Brain Res. 37, 294–300 (1972).
Kriebel, M.E., Bennett, M.V., Waxman, S.G. & Pappas, G.D. Oculomotor neurons in fish: electrotonic coupling and multiple sites of impulse initiation. Science 166, 520–524 (1969).
Korn, H. & Bennett, M.V. Vestibular nystagmus and teleost oculomotor neurons: functions of electrotonic coupling and dendritic impulse initiation. J. Neurophysiol. 38, 430–451 (1975).
Haag, J. & Borst, A. Amplification of high-frequency synaptic inputs by active dendritic membrane processes. Nature 379, 639–641 (1996).
Hausen, K. Motion sensitive interneurons in the optomotor system of the fly. II. The horizontal cells: Receptive field organization and response characteristics. Biol. Cybern. 46, 67–79 (1982).
Haag, J., Egelhaaf, M. & Borst, A. Dendritic integration of motion information in visual interneurons of the blowfly. Neurosci. Lett. 140, 173–176 (1992).
Gabbiani, F., Krapp, H.G., Koch, C. & Laurent, G. Multiplicative computation in a visual neuron sensitive to looming. Nature 420, 320–324 (2002).
Gabbiani, F., Krapp, H.G. & Laurent, G. Computation of object approach by wide-field, motion-sensitive neuron. J. Neurosci. 19, 1122–1141 (1998).
Jazayeri, M. & Movshon, J.A. Optimal representation of sensory information by neural populations. Nat. Neurosci. 9, 690–696 (2006).
Acknowledgements
We thank Y. Choe for useful comments on an early version of the manuscript, D. Spavieri Jr. for helpful discussions and R. Gleich for technical assistance. This study was supported by the Max Planck Society and by the Bernstein Center for Computational Neuroscience. Y.M.E. was partially supported by a Minerva Foundation fellowship from the Max Planck Society.
Author information
Authors and Affiliations
Contributions
Y.M.E., J.H. and A.B. collectively designed the experiments; Y.M.E. performed the experiments and simulations and evaluated the data; and Y.M.E., J.H. and A.B. collectively wrote the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6, Supplementary Table 1 and Supplementary Methods (PDF 597 kb)
Rights and permissions
About this article
Cite this article
Elyada, Y., Haag, J. & Borst, A. Different receptive fields in axons and dendrites underlie robust coding in motion-sensitive neurons. Nat Neurosci 12, 327–332 (2009). https://doi.org/10.1038/nn.2269
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2269
This article is cited by
-
Non-uniform weighting of local motion inputs underlies dendritic computation in the fly visual system
Scientific Reports (2018)
-
Electrical synapses convey orientation selectivity in the mouse retina
Nature Communications (2017)
-
Subcellular mapping of dendritic activity in optic flow processing neurons
Journal of Comparative Physiology A (2014)
-
Disentangling the functional consequences of the connectivity between optic-flow processing neurons
Nature Neuroscience (2012)
-
Bio-inspired visual ego-rotation sensor for MAVs
Biological Cybernetics (2012)