Abstract
Natural proteins often rely on the disulfide bond to covalently link side chains. Here we genetically introduce a new type of covalent bond into proteins by enabling an unnatural amino acid to react with a proximal cysteine. We demonstrate the utility of this bond for enabling irreversible binding between an affibody and its protein substrate, capturing peptide-protein interactions in mammalian cells, and improving the photon output of fluorescent proteins.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Accession codes
Change history
21 November 2013
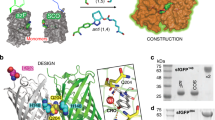
In the version of this article initially published, in Figure 2e, lanes 6 and 8 should have been labeled "Fact," not "Ffact." In the legend for this figure, the sentence "N3 indicates cysteine..." should have read "C3 indicates cysteine...." The errors have been corrected in the HTML and PDF versions of the article.
References
Sevier, C.S. & Kaiser, C.A. Nat. Rev. Mol. Cell Biol. 3, 836–847 (2002).
Liu, H. & May, K. MAbs 4, 17–23 (2012).
Berkmen, M. Protein Expr. Purif. 82, 240–251 (2012).
Kang, H.J. & Baker, E.N. Trends Biochem. Sci. 36, 229–237 (2011).
Liu, C.C. & Schultz, P.G. Annu. Rev. Biochem. 79, 413–444 (2010).
Li, X. & Liu, D.R. Angew. Chem. Int. Edn. Engl. 43, 4848–4870 (2004).
Drahl, C., Cravatt, B.F. & Sorensen, E.J. Angew. Chem. Int. Edn. Engl. 44, 5788–5809 (2005).
Chmura, A.J., Orton, M.S. & Meares, C.F. Proc. Natl. Acad. Sci. USA 98, 8480–8484 (2001).
Cravatt, B.F., Wright, A.T. & Kozarich, J.W. Annu. Rev. Biochem. 77, 383–414 (2008).
Ahmed, N.K. et al. Biochem. Pharmacol. 44, 1201–1207 (1992).
Cohen, M.S., Zhang, C., Shokat, K.M. & Taunton, J. Science 308, 1318–1321 (2005).
Bennett, B.D. et al. Nat. Chem. Biol. 5, 593–599 (2009).
Högbom, M., Eklund, M., Nygren, P.A. & Nordlund, P. Proc. Natl. Acad. Sci. USA 100, 3191–3196 (2003).
Holm, L., Moody, P. & Howarth, M. J. Biol. Chem. 284, 32906–32913 (2009).
Wang, L., Zhang, Z., Brock, A. & Schultz, P.G. Proc. Natl. Acad. Sci. USA 100, 56–61 (2003).
Perrin, M.H. & Vale, W.W. Ann. NY Acad. Sci. 885, 312–328 (1999).
Shu, X.K., Wang, L., Colip, L., Kallio, K. & Remington, S.J. Protein Sci. 18, 460–466 (2009).
Shcherbo, D. et al. Biochem. J. 418, 567–574 (2009).
Zhang, Z., Wang, L., Brock, A. & Schultz, P.G. Angew. Chem. Int. Edn. 41, 2840–2842 (2002).
Alken, M. et al. Biochem. J. 390, 455–464 (2005).
Lacey, V.K. et al. Angew. Chem. Int. Edn. Engl. 50, 8692–8696 (2011).
Wang, L., Jackson, W.C., Steinbach, P.A. & Tsien, R.Y. Proc. Natl. Acad. Sci. USA 101, 16745–16749 (2004).
Coin, I., Perrin, M.H., Vale, W.W. & Wang, L. Angew. Chem. Int. Edn. Engl. 50, 8077–8081 (2011).
Takimoto, J.K., Adams, K.L., Xiang, Z. & Wang, L. Mol. Biosyst. 5, 931–934 (2009).
Wang, W. et al. Nat. Neurosci. 10, 1063–1072 (2007).
Takimoto, J.K., Dellas, N., Noel, J.P. & Wang, L. ACS Chem. Biol. 6, 733–743 (2011).
Acknowledgements
We thank J. Xu for help with the NMR measurements, M. Beyermann (Leibniz Institute of Molecular Pharmacology, Germany) for synthesizing the Cys-Ucn-1 analogs, and the Vale laboratory (Salk Institute) for the polyclonal rabbit anti-urocortin. H.R. was partially funded by the Nomis Postdoctoral Fellowship. I.C. was supported by a Marie Curie fellowship from the European Commission within the 7th framework program. L.W. acknowledges support from the California Institute for Regenerative Medicine (RN1-00577-1) and US National Institutes of Health (1DP2OD004744-01, P30CA014195).
Author information
Authors and Affiliations
Contributions
Z.X. designed and synthesized the Uaa, tested the reaction, analyzed the data and wrote the manuscript; H.R. performed affibody-Z expression and complex formation, expressed and purified fluorescent proteins, measured quantum yields, analyzed the data and wrote the method section; Y.S.H. and H.C. performed single-molecule imaging, analyzed the data and wrote the single-molecule section. I.C. performed the CRF-R1 experiments and analyzed the data. J.W. characterized Uaa incorporation by MS, analyzed the data and wrote the MS section; L.W. conceived and directed the project, analyzed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
J.W. is an employee of Jadebio, Inc., which is a contract research organization providing mass spectrometry–based protein analysis.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4, Supplementary Table 1, Supplementary Note, Supplementary Results and Supplementary Methods (PDF 3679 kb)
Rights and permissions
About this article
Cite this article
Xiang, Z., Ren, H., Hu, Y. et al. Adding an unnatural covalent bond to proteins through proximity-enhanced bioreactivity. Nat Methods 10, 885–888 (2013). https://doi.org/10.1038/nmeth.2595
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.2595
This article is cited by
-
Genetically encoded chemical crosslinking of carbohydrate
Nature Chemistry (2023)
-
Association of CTLA-4 and IL-4 polymorphisms in viral induced liver cancer
BMC Cancer (2022)
-
Homodimerized cytoplasmic domain of PD-L1 regulates its complex glycosylation in living cells
Communications Biology (2022)
-
NeissLock provides an inducible protein anhydride for covalent targeting of endogenous proteins
Nature Communications (2021)
-
Efficient yeast surface-display of novel complex synthetic cellulosomes
Microbial Cell Factories (2018)