Abstract
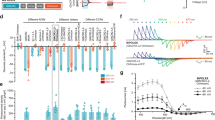
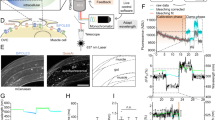
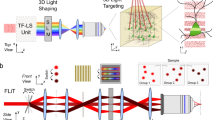
The ability to optically excite or silence specific cells using optogenetics has become a powerful tool to interrogate the nervous system. Optogenetic experiments in small organisms have mostly been performed using whole-field illumination and genetic targeting, but these strategies do not always provide adequate cellular specificity. Targeted illumination can be a valuable alternative but it has only been shown in motionless animals without the ability to observe behavior output. We present a real-time, multimodal illumination technology that allows both tracking and recording the behavior of freely moving C. elegans while stimulating specific cells that express channelrhodopsin-2 or MAC. We used this system to optically manipulate nodes in the C. elegans touch circuit and study the roles of sensory and command neurons and the ultimate behavioral output. This technology enhances our ability to control, alter, observe and investigate how neurons, muscles and circuits ultimately produce behavior in animals using optogenetics.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Zemelman, B., Nesnas, N., Lee, G. & Miesenbock, G. Photochemical gating of heterologous ion channels: Remote control over genetically designated populations of neurons. Proc. Natl. Acad. Sci. USA 100, 1352–1357 (2003).
Nagel, G. et al. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc. Natl. Acad. Sci. USA 100, 13940–13945 (2003).
Banghart, M., Borges, K., Isacoff, E., Trauner, D. & Kramer, R. Light-activated ion channels for remote control of neuronal firing. Nat. Neurosci. 7, 1381–1386 (2004).
Boyden, E., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268 (2005).
Nagel, G. et al. Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr. Biol. 15, 2279–2284 (2005).
Szobota, S. et al. Remote control of neuronal activity with a light-gated glutamate receptor. Neuron 54, 535–545 (2007).
Zhang, F. et al. Multimodal fast optical interrogation of neural circuitry. Nature 446, 633–634 (2007).
Chow, B.Y. et al. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature 463, 98–102 (2010).
Schroll, C. et al. Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae. Curr. Biol. 16, 1741–1747 (2006).
Arenkiel, B. et al. In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2. Neuron 54, 205–218 (2007).
Huber, D. et al. Sparse optical microstimulation in barrel cortex drives learned behaviour in freely moving mice. Nature 451, 61–67 (2008).
Wyart, C. et al. Optogenetic dissection of a behavioural module in the vertebrate spinal cord. Nature 461, 407–410 (2009).
Brenner, S. Genetics of Caenorhabditis elegans. Genetics 77, 71–94 (1974).
White, J., Southgate, E., Thomson, J. & Brenner, S. The structure of the nervous-system of the nematode Caenorhabditis elegans. Phil. Trans. R. Soc. Lond. B 314, 1–340 (1986).
Liewald, J.F. et al. Optogenetic analysis of synaptic function. Nat. Methods 5, 895–902 (2008).
Mahoney, T. et al. Intestinal signaling to GABAergic neurons regulates a rhythmic behavior in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 105, 16350–16355 (2008).
Liu, Q., Hollopeter, G. & Jorgensen, E. Graded synaptic transmission at the Caenorhabditis elegans neuromuscular junction. Proc. Natl. Acad. Sci. USA 106, 10823–10828 (2009).
Aravanis, A. et al. An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J. Neural Eng. 4, S143–S156 (2007).
Gradinaru, V. et al. Targeting and readout strategies for fast optical neural control in vitro and in vivo. J. Neurosci. 27, 14231–14238 (2007).
Guo, Z.V., Hart, A.C. & Ramanathan, S. Optical interrogation of neural circuits in Caenorhabditis elegans. Nat. Methods 6, 891–847 (2009).
Holden-Dye, L. & Walker, R.J. Anthelmintic drugs. in WormBook (ed. The C. elegans Research Community) (02 November 2007).
Chalfie, M. et al. The neural circuit for touch sensitivity in Caenorhabditis elegans. J. Neurosci. 5, 956–964 (1985).
Goodman, M.B. Mechanosensation. in WormBook (ed. The C. elegans Research Community) (6 January 2006).
Kaplan, J. & Horvitz, H. A dual mechanosensory and chemosensory neuron in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 90, 2227–2231 (1993).
Wicks, S.R. & Rankin, C.H. Integration of mechanosensory stimuli in Caenorhabditis elegans. J. Neurosci. 15, 2434–2444 (1995).
Brockie, P.J. & Maricq, A.V. Ionotropic glutamate receptors: genetics, behavior and electrophysiology. in WormBook (ed. The C. elegans Research Community) (19 January 2006).
Park, S.J., Goodman, M.B. & Pruitt, B.L. Analysis of nematode mechanics by piezoresistive displacement clamp. Proc. Natl. Acad. Sci. USA 104, 17376–17381 (2007).
Mellem, J.E., Brockie, P.J., Madsen, D.M. & Maricq, A.V. Action potentials contribute to neuronal signaling in C. elegans. Nat. Neurosci. 11, 865–867 (2008).
Gray, J.M., Hill, J.J. & Bargmann, C.I. A circuit for navigation in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 102, 3184–3191 (2005).
Stephens, G.J., Johnson-Kerner, B., Bialek, W. & Ryu, W.S. Dimensionality and dynamics in the behavior of C. elegans. PLoS Comp. Bio. 4, e1000028 (2008).
Geng, W., Cosman, P., Berry, C.C., Feng, Z. & Schafer, W.R. Automatic tracking, feature extraction and classification of C. elegans phenotypes. IEEE Trans. Biomed. Eng. 51, 1811–1820 (2004).
Ramot, D., Johnson, B.E., Berry, T.L., Carnell, L. & Goodman, M.B. The parallel worm tracker: a platform for measuring average speed and drug-induced paralysis in nematodes. PLoS ONE 3, e2208 (2008).
Feng, Z., Cronin, C.J., Wittig, J.H. Jr., Sternberg, P.W. & Schafer, W.R. An imaging system for standardized quantitative analysis of C. elegans behavior. BMC Bioinformatics 5, 115 (2004).
Acknowledgements
We thank members of the Caenorhabditis Genetic Center, W. Schafer and Y. Tanizawa (Medical Research Council-Laboratory of Molecular Biology, Cambridge, UK) and E. Boyden (Massachusetts Institute of Technology) for reagents; the US National Institutes of Health (H.L.), Alfred P. Sloan Foundation (H.L.), the Human Frontier Science Program Organization—HFSPO (S.J.H.), the Deutsche Forschungsgemeinschaft, grants GO1011/2-1, SFB807-P11, FOR1279-P1 and Cluster of Excellence Frankfurt, Macromolecular Complexes (A.G.) for funding; and K. Erbguth for discussions. We also thank J. Andrews and B. Parker in our machine shop.
Author information
Authors and Affiliations
Contributions
J.N.S., M.M.C., A.G. and H.L. designed the experiments. J.N.S. and M.M.C. wrote the software. J.N.S. constructed the illumination system, performed experiments and analyzed the data. S.J.H., S.W. and C.S. contributed to reagents and provided valuable discussions. J.N.S., M.M.C., S.J.H., A.G. and H.L. prepared the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 and Supplementary Notes 1–2 (PDF 779 kb)
Supplementary Video 1
Using the dorsal coiling effect and head illumination to dictate the locomotive path of an animal expressing ChR2 in the cholinergic motor neurons. False color recreation was overlaid to show stimulation location and timing. (MOV 175 kb)
Supplementary Video 2
Direct muscular control of a paralyzed worm using light. Worms expressing ChR2 in the muscle cells were paralyzed with an ivermectin solution (0.01 mg ml-1) and were controlled using structured illumination. False color recreation was overlaid to show stimulation location and timing. (MOV 663 kb)
Supplementary Video 3
Illumination line scans performed on freely behaving pmec-4::ChR2 animals eliciting acceleration or reversal behavior depending on the location of illumination. The line scan travels from posterior to anterior and, in separate experiments, anterior to posterior at ~100 μm s-1. Simultaneous blue and red light was used in order to visualize the location of illumination. (MOV 1194 kb)
Supplementary Video 4
Using localized light stimulation to excite anterior or posterior gentle touch neurons (in animals carrying pmec-4::ChR2), and anterior or posterior command interneurons (in animals carrying pglr-1::ChR2). False color recreation was overlaid to show stimulation location and timing. (MOV 2109 kb)
Supplementary Video 5
Examples of four response states ('NR', 'Sl/P', 'r', 'R') to optical stimulation of the anterior gentle touch neurons (pmec-4::ChR2). False color recreation was overlaid to show stimulation location and timing. (MOV 1820 kb)
Supplementary Video 6
Demonstration of complex illumination patterns to investigate sensory integration in pmec-4::ChR2 animals: light pulses of gradually increasing intensity were delivered to the anterior touch neurons, and in a separate experiment the same pulses delivered anteriorly while the posterior touch neurons were continuously illuminated. False color recreation was overlaid to show stimulation location and timing. (MOV 1399 kb)
Supplementary Video 7
At certain anterior and posterior illumination intensities the behavior response (forward and reverse locomotion) alternate (in animals carrying pmec-4::ChR2). False color recreation was overlaid to show stimulation location and timing. (MOV 393 kb)
Supplementary Video 8
Using two-color, spatially distinct optical stimuli to rapidly curtail touch avoidance behavior in animals carrying pmec-4::ChR2 and plgr-1::MAC::mCherry. Also shown is the inhibition of a spontaneous reversal, demonstrating that natural, and not merely optogenetically generated reversals, can be inhibited. False color recreation was overlaid to show stimulation location and timing. (MOV 2325 kb)
Supplementary Software
Software used for tracking and illumination control. (ZIP 3483 kb)
Rights and permissions
About this article
Cite this article
Stirman, J., Crane, M., Husson, S. et al. Real-time multimodal optical control of neurons and muscles in freely behaving Caenorhabditis elegans. Nat Methods 8, 153–158 (2011). https://doi.org/10.1038/nmeth.1555
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.1555
This article is cited by
-
A micro-LED array based platform for spatio-temporal optogenetic control of various cardiac models
Scientific Reports (2023)
-
An adaptive tracking illumination system for optogenetic control of single bacterial cells
Applied Microbiology and Biotechnology (2022)
-
An economical and highly adaptable optogenetics system for individual and population-level manipulation of Caenorhabditis elegans
BMC Biology (2021)