Abstract
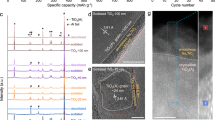
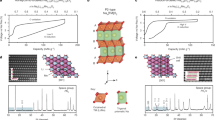
In contrast to monovalent lithium or sodium ions, the reversible insertion of multivalent ions such as Mg2+ and Al3+ into electrode materials remains an elusive goal. Here, we demonstrate a new strategy to achieve reversible Mg2+ and Al3+ insertion in anatase TiO2, achieved through aliovalent doping, to introduce a large number of titanium vacancies that act as intercalation sites. We present a broad range of experimental and theoretical characterizations that show a preferential insertion of multivalent ions into titanium vacancies, allowing a much greater capacity to be obtained compared to pure TiO2. This result highlights the possibility to use the chemistry of defects to unlock the electrochemical activity of known materials, providing a new strategy for the chemical design of materials for practical multivalent batteries.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Dunn, B., Kamath, H. & Tarascon, J.-M. Electrical energy storage for the grid: a battery of choices. Science 334, 928–935 (2011).
Armand, M. & Tarascon, J. M. Building better batteries. Nature 451, 652–657 (2008).
Tarascon, J. M. & Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 414, 359–367 (2001).
Ponrouch, A., Frontera, C., Barde, F. & Palacin, M. R. Towards a calcium-based rechargeable battery. Nat. Mater. 15, 169–172 (2016).
Aurbach, D. et al. Prototype systems for rechargeable magnesium batteries. Nature 407, 724–727 (2000).
Aurbach, D. et al. Progress in rechargeable magnesium battery technology. Adv. Mater. 19, 4260–4267 (2007).
Yoo, H. D. et al. Mg rechargeable batteries: an on-going challenge. Energy Environ. Sci. 6, 2265–2279 (2013).
Lapidus, S. H. et al. Solvation structure and energetics of electrolytes for multivalent energy storage. Phys. Chem. Chem. Phys. 16, 21941–21945 (2014).
Lin, M.-C. et al. An ultrafast rechargeable aluminium-ion battery. Nature 520, 324–328 (2015).
Elia, G. A. et al. An overview and future perspectives of aluminum batteries. Adv. Mater. 28, 7564–7579 (2016).
Ling, C., Banerjee, D. & Matsui, M. Study of the electrochemical deposition of Mg in the atomic level: Why it prefers the non-dendritic morphology. Electrochim. Acta 76, 270–274 (2012).
Mohtadi, R. & Mizuno, F. Magnesium batteries: current state of the art, issues and future perspectives. Beilstein J. Nanotech. 5, 1291–1311 (2014).
Levi, E., Gofer, Y. & Aurbach, D. On the way to rechargeable Mg batteries: the challenge of new cathode materials. Chem. Mater. 22, 860–868 (2010).
Huie, M. M., Bock, D. C., Takeuchi, E. S., Marschilok, A. C. & Takeuchi, K. J. Cathode materials for magnesium and magnesium-ion based batteries. Coord. Chem. Rev. 287, 15–27 (2015).
Rong, Z. et al. Materials design rules for multivalent ion mobility in intercalation structures. Chem. Mater. 27, 6016–6021 (2015).
Ling, C. & Mizuno, F. Phase stability of post-spinel compound AMn2O4(A = Li, Na, or Mg) and its application as a rechargeable battery cathode. Chem. Mater. 25, 3062–3071 (2013).
Wagemaker, M., Kentgens, A. P. M. & Mulder, F. M. Equilibrium lithium transport between nanocrystalline phases in intercalated TiO2 anatase. Nature 418, 397–399 (2002).
Borghols, W. J. H. et al. The electronic structure and ionic diffusion of nanoscale LiTiO2 anatase. Phys. Chem. Chem. Phys. 11, 5742–5748 (2009).
Ren, Y., Hardwick, L. J. & Bruce, P. G. Lithium intercalation into mesoporous anatase with an ordered 3D pore structure. Angew. Chem. Int. Ed. 49, 2570–2574 (2010).
Li, W. et al. High substitution rate in TiO2 anatase nanoparticles with cationic vacancies for fast lithium storage. Chem. Mater. 27, 5014–5019 (2015).
Su, S. et al. A novel rechargeable battery with a magnesium anode, a titanium dioxide cathode, and a magnesium borohydride/tetraglyme electrolyte. Chem. Commun. 51, 2641–2644 (2015).
Zhang, M., MacRae, A. C., Liu, H. & Meng, Y. S. Communication—investigation of anatase-TiO2 as an efficient electrode material for magnesium-ion batteries. J. Electrochem. Soc. 163, A2368–A2370 (2016).
Liu, S. et al. Aluminum storage behavior of anatase TiO2 nanotube arrays in aqueous solution for aluminum ion batteries. Energy Environ. Sci. 5, 9743–9746 (2012).
Legrain, F., Malyi, O. & Manzhos, S. Insertion energetics of lithium, sodium, and magnesium in crystalline and amorphous titanium dioxide: a comparative first-principles study. J. Power Sources 278, 197–202 (2015).
Li, W., Body, M., Legein, C., Borkiewicz, O. J. & Dambournet, D. Atomic insights into nanoparticle formation of hydroxyfluorinated anatase featuring titanium vacancies. Inorg. Chem. 55, 7182–7187 (2016).
Grimaud, A. et al. Activation of surface oxygen sites on an iridium-based model catalyst for the oxygen evolution reaction. Nat. Energy 2, 16189 (2016).
Wagemaker, M., Borghols, W. J. H. & Mulder, F. M. Large impact of particle size on insertion reactions. A case for anatase LixTiO2 . J. Am. Chem. Soc. 129, 4323–4327 (2007).
Wu, N. et al. A highly reversible, low-strain Mg-ion insertion anode material for rechargeable Mg-ion batteries. NPG Asia Mater. 6, e120 (2014).
Gu, Y., Katsura, Y., Yoshino, T., Takagi, H. & Taniguchi, K. Rechargeable magnesium-ion battery based on a TiSe2-cathode with d − p orbital hybridized electronic structure. Sci. Rep. 5, 12486 (2015).
Lee, B. et al. Investigation on the structural evolutions during the insertion of aluminum ions into Mo6S8 Chevrel phase. J. Electrochem. Soc. 163, A1070–A1076 (2016).
Wang, W. et al. A new cathode material for super-valent battery based on aluminium ion intercalation and deintercalation. Sci. Rep. 3, 3383 (2013).
Le, D. B. et al. Intercalation of polyvalent cations into V2O5 aerogels. Chem. Mater. 10, 682–684 (1998).
Chapman, K. W. Emerging operando and X-ray pair distribution function methods for energy materials development. MRS Bull. 41, 231–240 (2016).
Stoyanov, E., Langenhorst, F. & Steinle-Neumann, G. The effect of valence state and site geometry on Ti L3,2 and O K electron energy-loss spectra of TixOy phases. Am. Mineral. 92, 577–586 (2007).
Sadoc, A. et al. NMR parameters in alkali, alkaline Earth and rare Earth fluorides from first principle calculations. Phys. Chem. Chem. Phys. 13, 18539–18550 (2011).
Baur, W. H. & Khan, A. A. Rutile-type compounds. IV. SiO2, GeO2 and a comparison with other rutile-type structures. Acta Cryst. B 27, 2133–2139 (1971).
Fattakhova, D., Kavan, L. & Krtil, P. Lithium insertion into titanium dioxide (anatase) electrodes: microstructure and electrolyte effects. J. Solid State Electrochem. 5, 196–204 (2001).
Wen, C. J., Boukamp, B. A., Huggins, R. A. & Weppner, W. Thermodynamic and mass transport properties of “LiAl”? J. Electrochem. Soc. 126, 2258–2266 (1979).
Van der Ven, A., Bhattacharya, J. & Belak, A. A. Understanding Li diffusion in Li-intercalation compounds. Acc. Chem. Res. 46, 1216–1225 (2013).
Wang, J., Polleux, J., Lim, J. & Dunn, B. Pseudocapacitive contributions to electrochemical energy storage in TiO2 (Anatase) nanoparticles. J. Phys. Chem. C 111, 14925–14931 (2007).
Emly, A. & Van der Ven, A. Mg intercalation in layered and spinel host crystal structures for Mg Batteries. Inorg. Chem. 54, 4394–4402 (2015).
Lee, J. et al. Unlocking the potential of cation-disordered oxides for rechargeable lithium batteries. Science 343, 519–522 (2014).
Hahn, B. P., Long, J. W. & Rolison, D. R. Something from nothing: enhancing electrochemical charge storage with cation vacancies. Acc. Chem. Res. 46, 1181–1191 (2013).
Kim, H.-S. et al. Oxygen vacancies enhance pseudocapacitive charge storage properties of MoO3−x . Nat. Mater. 16, 454–460 (2017).
Pernet, M., Strobel, P., Bonnet, B., Bordet, P. & Chabre, Y. Structural and electrochemical study of lithium insertion into γ-Fe2O3 . Solid State Ion. 66, 259–265 (1993).
Gao, P. et al. The critical role of point defects in improving the specific capacitance of δ-MnO2 nanosheets. Nat. Commun. 8, 14559 (2017).
Chupas, P. J. Rapid-acquisition pair distribution function (RA-PDF) analysis. J. Appl. Crystallogr. 36, 1342–1347 (2003).
Chupas, P. J., Chapman, K. W. & Lee, P. L. Applications of an amorphous silicon-based area detector for high-resolution, high-sensitivity and fast time-resolved pair distribution function measurements. J. Appl. Crystallogr. 40, 463–470 (2007).
Hammersley, A. P., Svensson, S. O., Hanfland, M., Fitch, A. N. & Hausermann, D. Two-dimensional detector software: from real detector to idealised image or two-theta scan. High Press. Res. 14, 235–248 (1996).
Qiu, X., Thompson, J. W. & Billinge, S. J. L. PDFgetX2: a GUI-driven program to obtain the pair distribution function from X-ray powder diffraction data. J. Appl. Crystallogr. 37, 678 (2004).
Farrow, C. L. et al. PDFfit2 and PDFgui: computer programs for studying nanostructure in crystals. J. Phys. Condens. Matter. 19, 335219 (2007).
Ishii, Y., Wickramasinghe, N. P. & Chimon, S. A new approach in 1D and 2D 13C high-resolution solid-state NMR spectroscopy of paramagnetic organometallic complexes by very fast magic-angle spinning. J. Am. Chem. Soc. 125, 3438–3439 (2003).
van Gorkom, L. C. M., Hook, J. M., Logan, M. B., Hanna, J. V. & Wasylishen, R. E. Solid-state lead-207 NMR of lead(II) nitrate: localized heating effects at high magic angle spinning speeds. Magn. Reson. Chem. 33, 791–795 (1995).
Bielecki, A. & Burum, D. P. Temperature dependence of 207Pb MAS spectra of solid lead nitrate. An accurate, sensitive thermometer for variable-temperature MAS. J. Magn. Reson. Ser. A 116, 215–220 (1995).
Massiot, D. et al. Modelling one- and two-dimensional solid-state NMR spectra. Magn. Reson. Chem. 40, 70–76 (2002).
Kresse, G. & Hafner, J. Norm-conserving and ultrasoft pseudopotentials for first-row and transition elements. J. Phys. Condens. Matter. 6, 8245–8257 (1994).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Perdew, J. P. et al. Restoring the density-gradient expansion for exchange in solids and surfaces. Phys. Rev. Lett. 100, 136406 (2008).
Morgan, B. J. & Watson, G. W. GGA + U description of lithium intercalation into anatase TiO2 . Phys. Rev. B 82, 144119 (2010).
Morgan, B. J. & Watson, G. W. Role of lithium ordering in the LixTiO2 anatase → titanate phase transition. J. Phys. Chem. Lett. 2, 1657–1661 (2011).
Morgan, B. J. & Madden, P. A. Lithium intercalation into TiO2(B): a comparison of LDA, GGA, and GGA + U density functional calculations. Phys. Rev. B 86, 035147 (2012).
Morgan, B. J. et al. Computational Dataset for “Reversible Magnesium and Aluminium-ions Insertion in Cation-Deficient Anatase TiO2” University of Bath Research Data Archive http://researchdata.bath.ac.uk/id/eprint/397 (2017).
Morgan, B. J. “vasppy - a Python suite for manipulating VASP files”, https://github.com/bjmorgan/vasppy (2017).
Acknowledgements
The research leading to these results has received funding from the French National Research Agency under Idex@Sorbonne University for the Future Investments programme (No. ANR-11-IDEX-0004-02) and by the German Federal Ministry of Education and Research (BMBF) through funding by the ‘Sino German TU9 network for electromobility’ under the grant reference number 16N11929. B.J.M. acknowledges support from the Royal Society (UF130329). This work made use of the ARCHER UK National Supercomputing Service (http://www.archer.ac.uk), via the membership of the UK’s HPC Materials Chemistry Consortium, which is funded by EPSRC (EP/L000202). The work done at the Advanced Photon Source, an Office of Science User Facility operated for the US Department of Energy (DOE) Office of Science by Argonne National Laboratory, was supported by the US DOE under Contract No. DE-AC02-06CH11357. H.G. and D.D. wish to thank the French fluorine network for continuous support. M.B. and C.L. would like to thank C. Jacquemmoz (IMMM) for help with solid-state NMR experiments.
Author information
Authors and Affiliations
Contributions
J.M., P.S. and D.D. conceived and coordinated the study. T.K., J.M., M.B., C.L., W.D., M.G., A.D., O.J.B., K.W.C., F.D., H.G., P.S. and D.D. carried out experimental work and data analysis. B.J.M. and M.S. conducted the computational study. All authors discussed the results and commented on the manuscript. J.M. and D.D. wrote the manuscript with the contributions of all co-authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1326 kb)
Rights and permissions
About this article
Cite this article
Koketsu, T., Ma, J., Morgan, B. et al. Reversible magnesium and aluminium ions insertion in cation-deficient anatase TiO2. Nature Mater 16, 1142–1148 (2017). https://doi.org/10.1038/nmat4976
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4976
This article is cited by
-
Improving rechargeable magnesium batteries through dual cation co-intercalation strategy
Nature Communications (2024)
-
Manipulating disorder within cathodes of alkali-ion batteries
Nature Reviews Chemistry (2024)
-
Superlattice cathodes endow cation and anion co-intercalation for high-energy-density aluminium batteries
Nature Communications (2024)
-
Constructing highly safe and long-life calcium ion batteries based on hydratedvanadium oxide cathodes featuring a pillar structure
Rare Metals (2024)
-
Influence of LiCl on the kinetics of Mg2+ insertion into TiO2 prepared by solid-state chemical reaction
Journal of Solid State Electrochemistry (2024)