Abstract
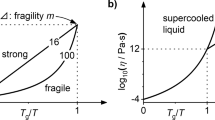
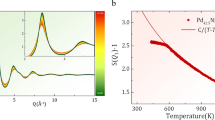
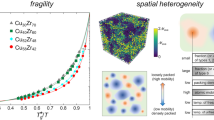
The dynamical behaviour of liquids is frequently characterized by the fragility, which can be defined from the temperature dependence of the shear viscosity, η (ref. 1). For a strong liquid, the activation energy for η changes little with cooling towards the glass transition temperature, Tg. The change is much greater in fragile liquids, with the activation energy becoming very large near Tg. While fragility is widely recognized as an important concept—believed, for example, to play an important role in glass formation2—the microscopic origin of fragility is poorly understood. Here, we present new experimental evidence showing that fragility reflects the strength of the repulsive part of the interatomic potential, which can be determined from the steepness of the pair distribution function near the hard-sphere cutoff. On the basis of an analysis of scattering data from ten different metallic alloy liquids, we show that stronger liquids have steeper repulsive potentials.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Angell, C. A. Perspective on the glass transition. J. Phys. Chem. Solids 49, 863–871 (1988).
Johnson, W. L., Na, J. H. & Demetriou, M. D. Quantifying the origin of metallic glass formation. Nat. Commun. 7, 10313 (2015).
Bennett, C., Polk, D. & Turnbull, D. Role of composition in metallic glass formation. Acta Metall. 19, 1295–1298 (1971).
Krausser, J., Samwer, K. H. & Zaccone, A. Interatomic repulsion softness directly controls the fragility of supercooled metallic melts. Proc. Natl Acad. Sci. USA 112, 13762–13767 (2015).
Yang, J. & Schweizer, K. S. Glassy dynamics and mechanical response in dense fluids of soft repulsive spheres. I. Activated relaxation, kinetic vitrification, and fragility. J. Chem. Phys. 134, 204908 (2011).
Mattsson, J. et al. Soft colloids make strong glasses. Nature 462, 83–86 (2009).
Casalini, R. The fragility of liquids and colloids and its relation to the softness of the potential. J. Chem. Phys. 137, 204904 (2012).
Casalini, R. & Roland, C. Why liquids are fragile. Phys. Rev. E 72, 031503 (2005).
Ngai, K. Relaxation and Diffusion in Complex Systems (Springer Science & Business Media, 2011).
Bordat, P., Affouard, F., Descamps, M. & Ngai, K. Does the interaction potential determine both the fragility of a liquid and the vibrational properties of its glassy state? Phys. Rev. Lett. 93, 105502 (2004).
Sengupta, S., Vasconcelos, F., Affouard, F. & Sastry, S. Dependence of the fragility of a glass former on the softness of interparticle interactions. J. Chem. Phys. 135, 194503 (2011).
Shi, Z., Debenedetti, P. G., Stillinger, F. H. & Ginart, P. Structure, dynamics, and thermodynamics of a family of potentials with tunable softness. J. Chem. Phys. 135, 084513 (2011).
Ozawa, M., Kim, K. & Miyazaki, K. Tuning pairwise potential can control the fragility of glass-forming liquids: from a tetrahedral network to isotropic soft sphere models. J. Stat. Mech. Theor. Exp. 2016, 074002 (2016).
Reith, D., Pütz, M. & Müller-Plathe, F. Deriving effective mesoscale potentials from atomistic simulations. J. Comput. Chem. 24, 1624–1636 (2003).
Hansen, J. P. & McDonald, I. R. Theory of Simple Liquids (Elsevier, 1990).
Shannon, R. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. 32, 751–767 (1976).
Lagogianni, A., Krausser, J., Evenson, Z., Samwer, K. & Zaccone, A. Unifying interatomic potential, g (r), elasticity, viscosity, and fragility of metallic glasses: analytical model, simulations, and experiments. J. Stat. Phys. 2016, 084001 (2016).
Iwashita, T., Nicholson, D. M. & Egami, T. Elementary excitations and crossover phenomenon in liquids. Phys. Rev. Lett. 110, 205504 (2013).
Soklaski, R., Tran, V., Nussinov, Z., Kelton, K. & Yang, L. A locally preferred structure characterises all dynamical regimes of a supercooled liquid. Philos. Mag. A 96, 1212–1227 (2016).
Kivelson, D., Kivelson, S. A., Zhao, X., Nussinov, Z. & Tarjus, G. A thermodynamic theory of supercooled liquids. Physica A 219, 27–38 (1995).
Blodgett, M., Egami, T., Nussinov, Z. & Kelton, K. Proposal for universality in the viscosity of metallic liquids. Sci. Rep. 5, 13837 (2015).
Jaiswal, A., Egami, T., Kelton, K. F., Schweizer, K. S. & Zhang, Y. Correlation between fragility and the arrhenius crossover phenomenon in metallic, molecular, and metwork liquids. Phys. Rev. Lett. 117, 205701 (2016).
Kelton, K. Kinetic and structural fragility—a correlation between structures and dynamics in metallic liquids and glasses. J. Phys. Condens. Matter 29, 023002 (2016).
Ito, K., Moynihan, C. T. & Angell, C. A. Thermodynamic determination of fragility in liquids and a fragile-to-strong liquid transition in water. Nature 398, 492–495 (1999).
Richert, R. & Angell, C. Dynamics of glass-forming liquids. V. On the link between molecular dynamics and configurational entropy. J. Chem. Phys. 108, 9016–9026 (1998).
Hu, Y.-C. et al. Thermodynamic scaling of glassy dynamics and dynamic heterogeneities in metallic glass-forming liquid. J. Chem. Phys. 145, 104503 (2016).
Mauro, N., Blodgett, M., Johnson, M., Vogt, A. & Kelton, K. A structural signature of liquid fragility. Nat. Commun. 5, 4616 (2014).
Rhim, W.-K., Ohsaka, K., Paradis, P.-F. & Spjut, R. E. Noncontact technique for measuring surface tension and viscosity of molten materials using high temperature electrostatic levitation. Rev. Sci. Instrum. 70, 2796–2801 (1999).
Mauro, N. & Kelton, K. A highly modular beamline electrostatic levitation facility, optimized for in situ high-energy X-ray scattering studies of equilibrium and supercooled liquids. Rev. Sci. Instrum. 82, 035114 (2011).
Bendert, J., Mauro, N. & Kelton, K. Pair distribution function analysis of X-ray diffraction from amorphous spheres in an asymmetric transmission geometry: application to a Zr58.5Cu15.6Ni12.8Al10.3Nb2.8 glass. J. Appl. Crystallogr. 46, 999–1007 (2013).
Johnson, M. Structural Evolution, Chemical Order, and Crystallization of Metallic Liquids and Glasses Electronic theses & dissertations, WUSTL (2015).
Sheng, H., Kramer, M., Cadien, A., Fujita, T. & Chen, M. Highly optimized embedded-atom-method potentials for fourteen fcc metals. Phys. Rev. B 83, 134118 (2011).
Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 117, 1–19 (1995).
Acknowledgements
We thank D. Robinson for his assistance with the high-energy X-ray diffraction studies at the APS and A. Agrawal, A. Gangopadhyay, K. Samwer and L. Yang for useful discussions. K.F.K. and C.E.P. gratefully acknowledge support by NASA under Grant No. NNX16AB52G and the National Science Foundation under Grant No. DMR 15-06553. M.S. gratefully acknowledges support by the Foundation for the Science and Technological Innovation Talent of Harbin (No. 2010RFQXG028). The synchrotron measurements were made on the Sector 6 beamline at the Advanced Photon Source. Use of the Advanced Photon Source is supported by the US Department of Energy, Basic Energy Science, Office of Science, under contract no. DE-AC02-06CH11357. Any opinions, findings and conclusions or recommendations expressed in this article are those of the author(s) and do not necessarily reflect the views of the National Science Foundation or of NASA.
Author information
Authors and Affiliations
Contributions
K.F.K. conceived of the study. K.F.K. and C.E.P. obtained the experimental data, and M.S. carried out the MD simulations. K.F.K. and C.E.P. analysed all experimental and MD data, and all authors contributed to writing and editing the document.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 349 kb)
Rights and permissions
About this article
Cite this article
Pueblo, C., Sun, M. & Kelton, K. Strength of the repulsive part of the interatomic potential determines fragility in metallic liquids. Nature Mater 16, 792–796 (2017). https://doi.org/10.1038/nmat4935
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4935
This article is cited by
-
Liquid-like atoms in dense-packed solid glasses
Nature Materials (2022)
-
Influence of the interatomic repulsive hardness on the microstructure and dynamics of CuZr metallic glasses
Journal of Molecular Modeling (2022)
-
Revealing hidden supercooled liquid states in Al-based metallic glasses by ultrafast scanning calorimetry: Approaching theoretical ceiling of liquid fragility
Science China Materials (2020)