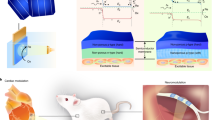
Abstract
Silicon-based materials have widespread application as biophysical tools and biomedical devices. Here we introduce a biocompatible and degradable mesostructured form of silicon with multi-scale structural and chemical heterogeneities. The material was synthesized using mesoporous silica as a template through a chemical vapour deposition process. It has an amorphous atomic structure, an ordered nanowire-based framework and random submicrometre voids, and shows an average Young’s modulus that is 2–3 orders of magnitude smaller than that of single-crystalline silicon. In addition, we used the heterogeneous silicon mesostructures to design a lipid-bilayer-supported bioelectric interface that is remotely controlled and temporally transient, and that permits non-genetic and subcellular optical modulation of the electrophysiology dynamics in single dorsal root ganglia neurons. Our findings suggest that the biomimetic expansion of silicon into heterogeneous and deformable forms can open up opportunities in extracellular biomaterial or bioelectric systems.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Leigh, C. Handbook of Porous Silicon 1st edn (Springer, 2014).
Sailor, M. J. Porous Silicon in Practice: Preparation, Characterization, and Applications (Wiley-VCH, 2012).
Kim, D.-H., Ghaffari, R., Lu, N. S. & Rogers, J. A. Flexible and stretchable electronics for biointegrated devices. Annu. Rev. Biomed. Eng. 14, 113–128 (2012).
Tian, B. Z. & Lieber, C. M. Synthetic nanoelectronic probes for biological cells and tissues. Annu. Rev. Anal. Chem. 6, 31–51 (2013).
Cogan, S. F. Neural stimulation and recording electrodes. Annu. Rev. Biomed. Eng. 10, 275–309 (2008).
Tasciotti, E. et al. Mesoporous silicon particles as a multistage delivery system for imaging and therapeutic applications. Nature Nanotech. 3, 151–157 (2008).
Chiappini, C. et al. Biodegradable silicon nanoneedles delivering nucleic acids intracellularly induce localized in vivo neovascularization. Nature Mater. 14, 532–539 (2015).
Gu, L. et al. In vivo time-gated fluorescence imaging with biodegradable luminescent porous silicon nanoparticles. Nature Commun. 4, 2326 (2013).
Kim, W., Ng, J. K., Kunitake, M. E., Conklin, B. R. & Yang, P. Interfacing silicon nanowires with mammalian cells. J. Am. Chem. Soc. 129, 7228–7229 (2007).
Hwang, S.-W. et al. A physically transient form of silicon electronics. Science 337, 1640–1644 (2012).
Park, J.-H. et al. Biodegradable luminescent porous silicon nanoparticles for in vivo applications. Nature Mater. 8, 331–336 (2009).
Kim, D.-H. et al. Dissolvable films of silk fibroin for ultrathin conformal bio-integrated electronics. Nature Mater. 9, 511–517 (2010).
Liu, J. et al. Syringe-injectable electronics. Nature Nanotech. 10, 629–636 (2015).
Zhang, A. Q. & Lieber, C. M. Nano-bioelectronics. Chem. Rev. 116, 215–257 (2016).
Zimmerman, J. F. et al. Free-standing kinked silicon nanowires for probing inter- and intracellular force dynamics. Nano Lett. 15, 5492–5498 (2015).
Wegst, U. G. K., Bai, H., Saiz, E., Tomsia, A. P. & Ritchie, R. O. Bioinspired structural materials. Nature Mater. 14, 23–36 (2015).
Chomski, E. & Ozin, G. A. Panoscopic silicon—a material for ‘all’ length scales. Adv. Mater. 12, 1071–1078 (2000).
Bao, Z. H. et al. Chemical reduction of three-dimensional silica micro-assemblies into microporous silicon replicas. Nature 446, 172–175 (2007).
Dai, F. et al. Bottom-up synthesis of high surface area mesoporous crystalline silicon and evaluation of its hydrogen evolution performance. Nature Commun. 5, 3605 (2014).
Hochbaum, A. I., Gargas, D., Hwang, Y. J. & Yang, P. Single crystalline mesoporous silicon nanowires. Nano Lett. 9, 3550–3554 (2009).
Qu, Y. et al. Electrically conductive and optically active porous silicon nanowires. Nano Lett. 9, 4539–4543 (2009).
Li, X. & Bohn, P. W. Metal-assisted chemical etching in HF/H(2)O(2) produces porous silicon. Appl. Phys. Lett. 77, 2572–2574 (2000).
Gordon, L. M. et al. Amorphous intergranular phases control the properties of rodent tooth enamel. Science 347, 746–750 (2015).
Ott, H. C. et al. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nature Med. 14, 213–221 (2008).
Gu, D. & Schuth, F. Synthesis of non-siliceous mesoporous oxides. Chem. Soc. Rev. 43, 313–344 (2014).
Wan, Y., Yang, H. F. & Zhao, D. Y. “Host-guest” chemistry in the synthesis of ordered nonsiliceous mesoporous materials. Acc. Chem. Res. 39, 423–432 (2006).
Arora, H. et al. Block copolymer self-assembly-directed single-crystal homo- and heteroepitaxial nanostructures. Science 330, 214–219 (2010).
Joo, S. H. et al. Ordered nanoporous arrays of carbon supporting high dispersions of platinum nanoparticles. Nature 412, 169–172 (2001).
Zhao, D. Y. et al. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 279, 548–552 (1998).
Richman, E. K., Kang, C. B., Brezesinski, T. & Tolbert, S. H. Ordered mesoporous silicon through magnesium reduction of polymer templated silica thin films. Nano Lett. 8, 3075–3079 (2008).
Tanaka, K., Maruyama, E., Shimada, T. & Okamoto, H. Amorphous Silicon 1st edn (Wiley, 1999).
Freund, L. B. & Suresh, S. Thin Film Materials: Stress, Defect Formation and Surface Evolution 1st edn (Cambridge Univ. Press, 2009).
Imperor-Clerc, M., Davidson, P. & Davidson, A. Existence of a microporous corona around the mesopores of silica-based SBA-15 materials templated by triblock copolymers. J. Am. Chem. Soc. 122, 11925–11933 (2000).
Minev, I. R. et al. Electronic dura mater for long-term multimodal neural interfaces. Science 347, 159–163 (2015).
Lanzani, G. Materials for bioelectronics: organic electronics meets biology. Nature Mater. 13, 775–776 (2014).
Gautieri, A., Vesentini, S., Redaelli, A. & Buehler, M. J. Hierarchical structure and nanomechanics of collagen microfibrils from the atomistic scale up. Nano Lett. 11, 757–766 (2011).
Picas, L., Rico, F. & Scheuring, S. Direct measurement of the mechanical properties of lipid phases in supported bilayers. Biophys. J. 102, L1–L3 (2012).
Han, D. X., Lorentzen, J. D., Weinberg-Wolf, J., McNeil, L. E. & Wang, Q. Raman study of thin films of amorphous-to-microcrystalline silicon prepared by hot-wire chemical vapor deposition. J. Appl. Phys. 94, 2930–2936 (2003).
Li, L. et al. Multifunctionality of chiton biomineralized armor with an integrated visual system. Science 350, 952–956 (2015).
Shapiro, M. G., Homma, K., Villarreal, S., Richter, C. P. & Bezanilla, F. Infrared light excites cells by changing their electrical capacitance. Nature Commun. 3, 736 (2012).
Carvalho-de-Souza, J. L. et al. Photosensitivity of neurons enabled by cell-targeted gold nanoparticles. Neuron 86, 207–217 (2015).
Sanders, A. W., Jeerage, K. M., Schwartz, C. L., Curtin, A. E. & Chiaramonti, A. N. Gold nanoparticle quantitation by whole cell tomography. ACS Nano 9, 11792–11799 (2015).
Liu, Y. et al. Dopamine-melanin colloidal nanospheres: an efficient near-infrared photothermal therapeutic agent for in vivo cancer therapy. Adv. Mater. 25, 1353–1359 (2013).
Kaplan, D. T. et al. Subthreshold dynamics in periodically stimulated squid giant axons. Phys. Rev. Lett. 76, 4074–4077 (1996).
Liu, Y., Ai, K. & Lu, L. Polydopamine and its derivative materials: synthesis and promising applications in energy, environmental, and biomedical fields. Chem. Rev. 114, 5057–5115 (2014).
Pan, L. et al. Hierarchical nanostructured conducting polymer hydrogel with high electrochemical activity. Proc. Natl Acad. Sci. USA 109, 9287–9292 (2012).
Ghezzi, D. et al. A hybrid bioorganic interface for neuronal photoactivation. Nature Commun. 2, 166 (2011).
Tee, B. C. K. et al. A skin-inspired organic digital mechanoreceptor. Science 350, 313–316 (2015).
Tian, B. Z. et al. Macroporous nanowire nanoelectronic scaffolds for synthetic tissues. Nature Mater. 11, 986–994 (2012).
Karzbrun, E., Tayar, A. M., Noireaux, V. & Bar-Ziv, R. H. Programmable on-chip DNA compartments as artificial cells. Science 345, 829–832 (2014).
Acknowledgements
This work is supported by the Air Force Office of Scientific Research (AFOSR FA9550-14-1-0175, FA9550-15-1-0285), the National Science Foundation (NSF CAREER, DMR-1254637; NSF MRSEC, DMR 1420709), the Searle Scholars Foundation, the National Institutes of Health (NIH GM030376), and the University of Chicago Start-up Fund. Atom-probe tomography was performed at the Northwestern University Center for Atom-Probe Tomography (NUCAPT), whose APT was purchased and upgraded with funding from NSF-MRI (DMR-0420532) and ONR-DURIP (N00014-0400798, N00014-0610539, N00014-0910781) grants. NUCAPT is a Research Facility at the Materials Research Center of Northwestern University, supported by the National Science Foundation’s MRSEC programme (grant number DMR-1121262). Instrumentation at NUCAPT was further upgraded by the Initiative for Sustainability and Energy at Northwestern (ISEN). This work made use of the JEOL JEM-ARM200CF and JEOL JEM-3010 TEM in the Electron Microscopy Service (Research Resources Center, UIC). The acquisition of the UIC JEOL JEM-ARM200CF was supported by an MRI-R2 grant from the National Science Foundation (DMR-0959470). A portion of this work was performed at the Center for Nanoscale Materials, a US Department of Energy, Office of Science, Office of Basic Energy Sciences User Facility under Contract No. DE-AC02-06CH11357. This research used the resources of the Advanced Photon Source, a US Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357. The authors thank D. Talapin, V. Srivastava, Y. Chen, J. Treger, T. Sun, Q. Guo, J. Jureller and R. N. S. Divan for providing technical support and stimulating discussions.
Author information
Authors and Affiliations
Contributions
Y.J. provided material design and synthesis. J.L.C.-d.-S. conducted lipid and neuron experiments Y.J., R.C.S.W., Z.L., D.I., X.Z., A.W.N., I.W.J., D.-J.L., Y.W., V.D.A., X.X., L.N. and D.N.S. performed material characterizations. Y.J., J.Y., R.C.S.W., D.E.W. and X.W. conducted biocompatibility and degradability studies in vitro and in vivo. Y.J. and R.C.S.W. carried out material data analysis. J.L.C.-d.-S., R.C.S.W., Y.J. and L.N. performed lipid and cell data analysis. Y.J. performed the COMSOL simulation. Y.J., R.C.S.W. and B.T. wrote the paper, and received comments and edits from all authors. B.T. and F.B. mentored the research.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 4813 kb)
Rights and permissions
About this article
Cite this article
Jiang, Y., Carvalho-de-Souza, J., Wong, R. et al. Heterogeneous silicon mesostructures for lipid-supported bioelectric interfaces. Nature Mater 15, 1023–1030 (2016). https://doi.org/10.1038/nmat4673
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4673
This article is cited by
-
Monolithic silicon for high spatiotemporal translational photostimulation
Nature (2024)
-
Bioinspired nanotransducers for neuromodulation
Nano Research (2024)
-
Neural modulation with photothermally active nanomaterials
Nature Reviews Bioengineering (2023)
-
Genetically targeted chemical assembly
Nature Reviews Bioengineering (2023)
-
Nanostructured germanium synthesized by high-pressure chemical vapor deposition in mesoporous silica templates
Journal of Materials Science: Materials in Electronics (2023)