Abstract
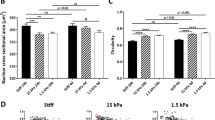
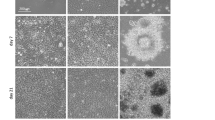
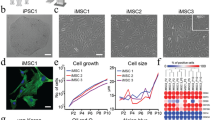
Since the discovery of induced pluripotent stem cells (iPSCs), numerous approaches have been explored to improve the original protocol, which is based on a two-dimensional (2D) cell-culture system. Surprisingly, nothing is known about the effect of a more biologically faithful 3D environment on somatic-cell reprogramming. Here, we report a systematic analysis of how reprogramming of somatic cells occurs within engineered 3D extracellular matrices. By modulating microenvironmental stiffness, degradability and biochemical composition, we have identified a previously unknown role for biophysical effectors in the promotion of iPSC generation. We find that the physical cell confinement imposed by the 3D microenvironment boosts reprogramming through an accelerated mesenchymal-to-epithelial transition and increased epigenetic remodelling. We conclude that 3D microenvironmental signals act synergistically with reprogramming transcription factors to increase somatic plasticity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Ben-Ze’ev, A., Farmer, S. R. & Penman, S. Protein synthesis requires cell-surface contact while nuclear events respond to cell shape in anchorage-dependent fibroblasts. Cell 21, 365–372 (1980).
Chen, C. S., Mrksich, M., Huang, S., Whitesides, G. M. & Ingber, D. E. Geometric control of cell life and death. Science 276, 1425–1428 (1997).
Chowdhury, F. et al. Material properties of the cell dictate stress-induced spreading and differentiation in embryonic stem cells. Nature Mater. 9, 82–88 (2010).
Engler, A. J., Sen, S., Sweeney, H. L. & Discher, D. E. Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 (2006).
Lutolf, M. P. & Hubbell, J. A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nature Biotechnol. 23, 47–55 (2005).
Singhvi, R. et al. Engineering cell shape and function. Science 264, 696–698 (1994).
Thomas, C. H., Collier, J. H., Sfeir, C. S. & Healy, K. E. Engineering gene expression and protein synthesis by modulation of nuclear shape. Proc. Natl Acad. Sci. USA 99, 1972–1977 (2002).
Bissell, M. J., Rizki, A. & Mian, I. S. Tissue architecture: the ultimate regulator of breast epithelial function. Curr. Opin. Cell Biol. 15, 753–762 (2003).
Roskelley, C. D., Desprez, P. Y. & Bissell, M. J. Extracellular matrix-dependent tissue-specific gene expression in mammary epithelial cells requires both physical and biochemical signal transduction. Proc. Natl Acad. Sci. USA 91, 12378–12382 (1994).
Streuli, C. H. et al. Laminin mediates tissue-specific gene expression in mammary epithelia. J. Cell. Biol. 129, 591–603 (1995).
Grant, P. A. A tale of histone modifications. Genome Biol. 2 http://dx.doi.org/10.1186/gb-2001-2-4-reviews0003 (2001).
Sancho-Martinez, I. & Izpisua Belmonte, J. C. Stem cells: surf the waves of reprogramming. Nature 493, 310–311 (2013).
Polo, J. M. et al. A molecular roadmap of reprogramming somatic cells into iPS cells. Cell 151, 1617–1632 (2012).
Hansson, J. et al. Highly coordinated proteome dynamics during reprogramming of somatic cells to pluripotency. Cell. Rep. 2, 1579–1592 (2012).
Downing, T. L. et al. Biophysical regulation of epigenetic state and cell reprogramming. Nature Mater. 12, 1154–1162 (2013).
Ehrbar, M. et al. Enzymatic formation of modular cell-instructive fibrin analogs for tissue engineering. Biomaterials 28, 3856–3866 (2007).
Ehrbar, M. et al. Biomolecular hydrogels formed and degraded via site-specific enzymatic reactions. Biomacromolecules 8, 3000–3007 (2007).
Ehrbar, M. et al. Elucidating the role of matrix stiffness in 3D cell migration and remodeling. Biophys. J. 100, 284–293 (2011).
Ranga, A. et al. 3D niche microarrays for systems-level analyses of cell fate. Nature Commun. 5, 4324 (2014).
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).
Lengner, C. J. et al. Oct4 expression is not required for mouse somatic stem cell self-renewal. Cell Stem Cell 1, 403–415 (2007).
Markoulaki, S. et al. Transgenic mice with defined combinations of drug-inducible reprogramming factors. Nature Biotechnol. 27, 169–171 (2009).
Hawkins, K., Mohamet, L., Ritson, S., Merry, C. L. & Ward, C. M. E-cadherin and, in its absence, N-cadherin promotes Nanog expression in mouse embryonic stem cells via STAT3 phosphorylation. Stem Cells 30, 1842–1851 (2012).
Gonzalez, B., Denzel, S., Mack, B., Conrad, M. & Gires, O. EpCAM is involved in maintenance of the murine embryonic stem cell phenotype. Stem Cells 27, 1782–1791 (2009).
Domogatskaya, A., Rodin, S., Boutaud, A. & Tryggvason, K. Laminin-511 but not -332, -111, or -411 enables mouse embryonic stem cell self-renewal in vitro. Stem Cells 26, 2800–2809 (2008).
Soteriou, D. et al. Comparative proteomic analysis of supportive and unsupportive extracellular matrix substrates for human embryonic stem cell maintenance. J. Biol. Chem. 288, 18716–18731 (2013).
Braam, S. R. et al. Recombinant vitronectin is a functionally defined substrate that supports human embryonic stem cell self-renewal via alphavbeta5 integrin. Stem Cells 26, 2257–2265 (2008).
Hayashi, Y. et al. Integrins regulate mouse embryonic stem cell self-renewal. Stem Cells 25, 3005–3015 (2007).
Marson, A. et al. Wnt signaling promotes reprogramming of somatic cells to pluripotency. Cell Stem Cell 3, 132–135 (2008).
Dupont, S. et al. Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183 (2011).
Lian, I. et al. The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Dev. 24, 1106–1118 (2010).
Aulicino, F., Theka, I., Ombrato, L., Lluis, F. & Cosma, M. P. Temporal perturbation of the Wnt signaling pathway in the control of cell reprogramming is modulated by TCF1. Stem Cell Rep. 2, 707–720 (2014).
Azzolin, L. et al. Role of TAZ as mediator of Wnt signaling. Cell 151, 1443–1456 (2012).
Li, R. et al. A mesenchymal-to-epithelial transition initiates and is required for the nuclear reprogramming of mouse fibroblasts. Cell Stem Cell 7, 51–63 (2010).
Samavarchi-Tehrani, P. et al. Functional genomics reveals a BMP-driven mesenchymal-to-epithelial transition in the initiation of somatic cell reprogramming. Cell Stem Cell 7, 64–77 (2010).
Schmidt, R. & Plath, K. The roles of the reprogramming factors Oct4, Sox2 and Klf4 in resetting the somatic cell epigenome during induced pluripotent stem cell generation. Genome Biol. 13, 251 (2012).
Koche, R. P. et al. Reprogramming factor expression initiates widespread targeted chromatin remodeling. Cell Stem Cell 8, 96–105 (2011).
Ang, Y. S. et al. Wdr5 mediates self-renewal and reprogramming via the embryonic stem cell core transcriptional network. Cell 145, 183–197 (2011).
Huangfu, D. et al. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nature Biotechnol. 26, 1269–1275 (2008).
Gerecht, S. et al. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proc. Natl Acad. Sci. USA 104, 11298–11303 (2007).
Lee, S. T. et al. Engineering integrin signaling for promoting embryonic stem cell self-renewal in a precisely defined niche. Biomaterials 31, 1219–1226 (2010).
Lee, S. T. et al. Long-term maintenance of mouse embryonic stem cell pluripotency by manipulating integrin signaling within 3D scaffolds without active Stat3. Biomaterials 33, 8934–8942 (2012).
Siti-Ismail, N., Bishop, A. E., Polak, J. M. & Mantalaris, A. The benefit of human embryonic stem cell encapsulation for prolonged feeder-free maintenance. Biomaterials 29, 3946–3952 (2008).
Lei, Y. & Schaffer, D. V. A fully defined and scalable 3D culture system for human pluripotent stem cell expansion and differentiation. Proc. Natl Acad. Sci. USA 110, E5039–5048 (2013).
Volpicelli, F. et al. Bdnf gene is a downstream target of Nurr1 transcription factor in rat midbrain neurons in vitro. J. Neurochem. 102, 441–453 (2007).
Di Stefano, B. et al. An ES-like pluripotent state in FGF-dependent murine iPS cells. PLoS ONE 5, e16092 (2010).
Acknowledgements
We thank M. Snyder and M. Knobloch for critical reading of the manuscript, A. Negro for polymer batch testing, D. Trono for providing Oct4–GFP and OKSM-doxy-inducible mice, M. Friedli for providing SFFV-OKSM lentiviral vector and for helpful discussion, M. Pluchinotta and P. Manti for the anti-E-Cad and anti-SMA antibodies, the EPFL Transgenic Core Facilities for generating chimaeric mice, and the EPFL Histology Core Facility for performing teratoma histology. This work was financially supported by the EU framework 7 HEALTH research programme PluriMes (http://www.plurimes.eu), the SystemsX.ch RTD project StoNets, an ERC grant (StG_311422) and a Swiss National Science Foundation Singergia grant (CRSII3_147684).
Author information
Authors and Affiliations
Contributions
M.P.L., Y.O. and M.C. designed the experiments and analysed the data. Y.O. and M.C. performed most of the experiments and statistical analyses. M.P.L. and M.C. wrote the manuscript. A.R. performed and analysed the HTS experiment and contributed to manuscript writing. Y.T. fabricated the microgroove platform. A.P. analysed teratoma assays.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 5815 kb)
Supplementary Information
Supplementary Information (XLSX 50 kb)
Supplementary Movie
Supplementary Movie (MP4 1357 kb)
Rights and permissions
About this article
Cite this article
Caiazzo, M., Okawa, Y., Ranga, A. et al. Defined three-dimensional microenvironments boost induction of pluripotency. Nature Mater 15, 344–352 (2016). https://doi.org/10.1038/nmat4536
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4536
This article is cited by
-
Development of three-dimensional (3D) spheroid culture system from rainbow trout kidney cell line (RTK) for in vitro production of fish viral pathogen
Aquaculture International (2024)
-
Mechanics of the cellular microenvironment as probed by cells in vivo during zebrafish presomitic mesoderm differentiation
Nature Materials (2023)
-
Thinking in 3 dimensions: philosophies of the microenvironment in organoids and organs-on-chip
History and Philosophy of the Life Sciences (2023)
-
Chemical strategies to engineer hydrogels for cell culture
Nature Reviews Chemistry (2022)
-
Actomyosin contractility as a mechanical checkpoint for cell state transitions
Scientific Reports (2022)