Abstract
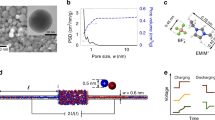
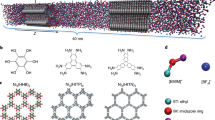
Supercapacitors have exceptional power density and cyclability but smaller energy density than batteries. Their energy density can be increased using ionic liquids and electrodes with subnanometre pores, but this tends to reduce their power density and compromise the key advantage of supercapacitors. To help address this issue through material optimization, here we unravel the mechanisms of charging subnanometre pores with ionic liquids using molecular dynamics simulations, navigated by a phenomenological model. We show that charging of ionophilic pores is a diffusive process, often accompanied by overfilling followed by de-filling. In sharp contrast to conventional expectations, charging is fast because ion diffusion during charging can be an order of magnitude faster than in the bulk, and charging itself is accelerated by the onset of collective modes. Further acceleration can be achieved using ionophobic pores by eliminating overfilling/de-filling and thus leading to charging behaviour qualitatively different from that in conventional, ionophilic pores.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
25 March 2014
In the version of this Article originally published, the e-mail address of Svyatoslav Kondrat was misspelt; it should have read ‘s.kondrat@fz-juelich.de’. This error has now been corrected in the online versions of the Article.
References
Conway, B. E. Electrochemical Capacitors: Scientific Fundamentals and Technological Applications (Kluwer, (1999).
Miller, J. R. & Simon, P. Materials science electrochemical capacitors for energy management. Science 321, 651–652 (2008).
Simon, P. & Gogotsi, Y. Materials for electrochemical capacitors. Nature Mater. 7, 845–854 (2008).
Chmiola, J. et al. Anomalous increase in carbon capacitance at pore sizes less than 1 nanometre. Science 313, 1760–1763 (2006).
Largeot, C. et al. Relation between the ion size and pore size for an electric double-layer capacitor. J. Am. Chem. Soc. 130, 2730–2731 (2008).
Lin, R. et al. Solvent effect on the ion adsorption from ionic liquid electrolyte into sub-nanometre carbon pores. Electrochim. Acta 54, 7025–7032 (2009).
Kondrat, S., P’erez, C. R., Presser, V., Gogotsi, Y. & Kornyshev, A. A. Effect of pore size and its dispersity on the energy storage in nanoporous supercapacitors. Energy Environ. Sci. 5, 6474–6479 (2012).
Simon, P. & Gogotsi, Y. Capacitive energy storage in nanostructured carbon electrolyte systems. Acc. Chem. Res. 46, 1094–1103 (2013).
Wang, H. et al. Interconnected carbon nanosheets derived from hemp for ultrafast supercapacitors with high energy. ACS Nano 7, 5131–5141 (2013).
Yoo, J. J. et al. Ultrathin planar graphene supercapacitors. Nano Lett. 11, 1423–1427 (2011).
Zhu, Y. et al. Carbon-based supercapacitors produced by activation of graphene. Science 332, 1537–1541 (2011).
Yang, X., Cheng, C., Wang, Y., Qiu, L. & Li, D. Liquid-mediated dense integration of graphene materials for compact capacitive energy storage. Science 341, 534–537 (2013).
Lukatskaya, M. R. et al. Cation intercalation and high volumetric capacitance of two-dimensional titanium carbide. Science 341, 1502–1505 (2013).
Huang, J., Sumpter, B. G. & Meunier, V. Theoretical model for nanoporous carbon supercapacitors. Angew. Chem. Int. Ed. 47, 520–524 (2008).
Shim, Y. & Kim, H. J. Nanoporous carbon supercapacitors in an ionic liquid: A computer simulation study. ACS Nano 4, 2345–2355 (2010).
Skinner, B., Chen, T., Loth, M. S. & Shklovskii, B. I. Theory of volumetric capacitance of an electric double-layer supercapacitor. Phys. Rev. E 83, 056102 (2011).
Kondrat, S. & Kornyshev, A. Superionic state in double-layer capacitors with nanoporous electrodes. J. Phys.: Condens. Matter 23, 022201 (2011).
Kondrat, S. & Kornyshev, A. Corrigendum: Superionic state in double-layer capacitors with nanoporous electrodes. J. Phys.: Condens. Matter 25, 119501 (2013).
Wu, P., Huang, J., Meunier, V., Sumpter, B. G. & Qiao, R. Complex capacitance scaling in ionic liquids-filled nanopores. ACS Nano 5, 9044–9051 (2011).
Feng, G. & Cummings, P. T. Supercapacitor capacitance exhibits oscillatory behaviour as a function of nanopore size. J. Phys. Chem. Lett. 2, 2859–2864 (2011).
Jiang, D. E., Jin, Z. H. & Wu, J. Z. Oscillation of capacitance inside nanopores. Nano Lett. 11, 5373–5377 (2011).
Merlet, C. et al. On the molecular origin of supercapacitance in nanoporous carbon electrodes. Nature Mater. 11, 306–310 (2012).
Xing, L., Vatamanu, J., Borodin, O. & Bedrov, D. On the atomistic nature of capacitance enhancement generated by ionic liquid electrolyte confined in subnanometre pores. J. Phys. Chem. Lett. 4, 132–140 (2013).
Merlet, C. et al. Highly confined ions store charge more efficiently in supercapacitors. Nature Commun. 4, 2701 (2013).
Brandt, A., Pohlmann, S., Varzi, A., Balducci, A. & Passerini, S. Ionic liquids in supercapacitors. MRS Bull. 38, 554–559 (2013).
Monk, J., Singh, R. & Hung, F. R. Effects of pore size and pore loading on the properties of ionic liquids confined inside nanoporous CMK-3 carbon materials. J. Phys. Chem. C 115, 3034–3042 (2011).
Rajput, N. N., Monk, J. & Hung, F. R. Structure and dynamics of an ionic liquid confined inside a charged slit graphitic nanopore. J. Phys. Chem. C 116, 14504–14513 (2012).
Perkin, S. Ionic liquids in confined geometries. Phys. Chem. Chem. Phys. 14, 5052–5062 (2012).
Bazant, M. Z., Thornton, K. & Ajdari, A. Diffuse-charge dynamics in electrochemical systems. Phys. Rev. E 70, 021506 (2004).
Biesheuvel, P. M. & Bazant, M. Z. Nonlinear dynamics of capacitive charging and desalination by porous electrodes. Phys. Rev. E 81, 031502 (2010).
Kondrat, S. & Kornyshev, A. Charging dynamics and optimization of nanoporous supercapacitors. J. Phys. Chem. C 117, 12399–12406 (2013).
Taberna, P. L., Simon, P. & Fauvarque, J. F. Electrochemical characteristics and impedance spectroscopy studies of carbon–carbon supercapacitors. J. Electrochem. Soc. 150, A292–A300 (2003).
Whitaker, S. Fundamental Principles of Heat Transfer (Pergamon Press, 1977).
Galantini, L. & Pavel, N. V. Collective diffusion and self-diffusion coefficients comparison to separate interactions and micellar size effects on ionic micelle diffusivities: Cylindrical micelles of sodium taurodeoxycholate. J. Chem. Phys. 118, 2865–2872 (2003).
Kilic, M. S., Bazant, M. Z. & Ajdari, A. Steric effects in the dynamics of electrolytes at large applied voltages ii modified Poisson–Nernst–Planck equations. Phys. Rev. E 75, 021503 (2007).
Iacob, C. et al. Enhanced charge transport in nano-confined ionic liquids. Soft Matter 8, 289–293 (2012).
Klahn, M., Seduraman, A. & Wu, P. A model for self-diffusion of guanidinium-based ionic liquids: a molecular simulation study. J. Phys. Chem. B 112, 13849–13861 (2008).
Tsai, W-Y. et al. Outstanding performance of activated graphene based supercapacitors in ionic liquid electrolyte from 50 to 80 C. Nano Energy 2, 403–411 (2013).
Fic, K., Lot, G. & Frackowiak, E. Effect of surfactants on capacitance properties of carbon electrodes. Electrochim. Acta 60, 206–212 (2011).
Mattia, D. & Gogotsi, Y. Review: Static and dynamic behaviour of liquids inside carbon nanotubes. Microfluid. Nanofluid. 5, 289–305 (2008).
Borukhov, I., Andelman, D. & Orland, H. Steric effects in electrolytes: A modified Poisson–Boltzmann equation. Phys. Rev. Lett. 79, 435–438 (1997).
Kondrat, S., Kornyshev, A., Stoeckli, F. & Centeno, T.A. The effect of dielectric permittivity on the capacitance of nanoporous electrodes. Electrochem. Comm. 34, 348–350 (2013).
Kornyshev, A. A., Ulstrup, J. & Vorotyntsev, M. A. The effect of the spatial dispersion of the dielectric permittivity on the capacitance of thin insulating films: Nonlinear dependence of the inverse capacitance on film thickness. Thin Solid Films 75, 105–118 (1981).
GNU Scientific Library. http://www.gnu.org/software/gsl/
Lindahl, E., Hess, B. & van der Spoel, D. Gromacs 3.0: A package for molecular simulation and trajectory analysis. J. Mol. Modell. 7, 306–331 (2001).
Raghunathan, A. V. & Aluru, N. R. Self-consistent molecular dynamics formulation for electric-field-mediated electrolyte transport through nanochannels. Phys. Rev. E 76, 011202 (2007).
Wu, P., Huang, J. S., Meunier, V., Sumpter, B. G. & Qiao, R. Voltage dependent charge storage modes and capacity in sub-nanometre pores. J. Phys. Chem. Lett. 3, 1732–1737 (2012).
Merlet, C. et al. Simulating supercapacitors: Can we model electrodes as constant charge surfaces? J. Phys. Chem. Lett. 4, 264–268 (2013).
Bussi, G., Donadio, D. & Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 126, 014101 (2007).
Frenkel, D. & Smith, B. Understanding Molecular Simulations (Academic, (1996).
Acknowledgements
We thank the Clemson-CCIT office and E. Duffy for providing computer facilities. R.Q. acknowledges the support of the NSF (CBET-1264578). S.K. and A.K. were supported by the Engineering and Physical Science Research Council via Grant EP/H004319/1. We are grateful to Y. Gogotsi, P. Simon, C. Pérez, J. Griffin, G. Oshanin and F. Stoeckli for fruitful discussions, and X. Jiang for technical assistance.
Author information
Authors and Affiliations
Contributions
A.A.K., R.Q. and S.K. designed the research. P.W. performed MD simulations and S.K. performed MFT calculations. The results were analysed jointly by R.Q., S.K., A.A.K. and P.W., and all participated in writing the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 499 kb)
Supplementary Information
Supplementary Movie 1 (MOV 488 kb)
Supplementary Information
Supplementary Movie 2 (MOV 30531 kb)
Supplementary Information
Supplementary Movie 3 (MOV 15960 kb)
Supplementary Information
Supplementary Movie 4 (MOV 29748 kb)
Rights and permissions
About this article
Cite this article
Kondrat, S., Wu, P., Qiao, R. et al. Accelerating charging dynamics in subnanometre pores. Nature Mater 13, 387–393 (2014). https://doi.org/10.1038/nmat3916
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat3916
This article is cited by
-
N-doped porous carbon nanofibers derived from N-rich cross-linked polymer for high-performance supercapacitors
Ionics (2024)
-
2D CdPS3-based versatile superionic conductors
Nature Communications (2023)
-
Flexible iontronics based on 2D nanofluidic material
Nature Communications (2022)
-
Carbon electrodes with ionophobic characteristics in organic electrolyte for high-performance electric double-layer capacitors
Science China Materials (2022)
-
Modeling galvanostatic charge–discharge of nanoporous supercapacitors
Nature Computational Science (2021)