Abstract
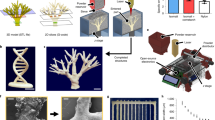
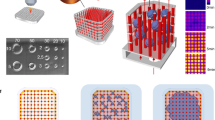
In the absence of perfusable vascular networks, three-dimensional (3D) engineered tissues densely populated with cells quickly develop a necrotic core1. Yet the lack of a general approach to rapidly construct such networks remains a major challenge for 3D tissue culture2,3,4. Here, we printed rigid 3D filament networks of carbohydrate glass, and used them as a cytocompatible sacrificial template in engineered tissues containing living cells to generate cylindrical networks that could be lined with endothelial cells and perfused with blood under high-pressure pulsatile flow. Because this simple vascular casting approach allows independent control of network geometry, endothelialization and extravascular tissue, it is compatible with a wide variety of cell types, synthetic and natural extracellular matrices, and crosslinking strategies. We also demonstrated that the perfused vascular channels sustained the metabolic function of primary rat hepatocytes in engineered tissue constructs that otherwise exhibited suppressed function in their core.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Radisic, M. et al. Medium perfusion enables engineering of compact and contractile cardiac tissue. Am. J. Physiol. Heart Circ. Physiol. 286, H507–H516 (2004).
Langer, R. & Vacanti, J. P. Tissue engineering. Science 260, 920–926 (1993).
Griffith, L. G. & Swartz, M. A. Capturing complex 3D tissue physiology in vitro. Nature Rev. Mol. Cell Biol. 7, 211–224 (2006).
Vunjak-Novakovic, G. & Scadden, D. T. Biomimetic platforms for human stem cell research. Cell Stem Cell 8, 252–261 (2011).
Schmidt-Nielsen, K. Scaling in biology: The consequences of size. J. Exp. Zool. 194, 287–307 (1975).
Golden, A. P. & Tien, J. Fabrication of microfluidic hydrogels using molded gelatin as a sacrificial element. Lab Chip 7, 720–725 (2007).
Ling, Y. et al. A cell-laden microfluidic hydrogel. Lab Chip 7, 756–762 (2007).
Choi, N. W. et al. Microfluidic scaffolds for tissue engineering. Nature Mater. 6, 908–915 (2007).
Cuchiara, M. P., Allen, A. C. B., Chen, T. M., Miller, J. S. & West, J. L. Multilayer microfluidic PEGDA hydrogels. Biomaterials 31, 5491–5497 (2010).
Visconti, R. P. et al. Towards organ printing: Engineering an intra-organ branched vascular tree. Exp. Opin. Biol. Therapy 10, 409–420 (2010).
Therriault, D., White, S. R. & Lewis, J. A. Chaotic mixing in three-dimensional microvascular networks fabricated by direct-write assembly. Nature Mater. 2, 265–271 (2003).
Bellan, L. M. et al. Fabrication of an artificial 3-dimensional vascular network using sacrificial sugar structures. Soft Matter 5, 1354–1357 (2009).
Wu, W. et al. Direct-write assembly of biomimetic microvascular networks for efficient fluid transport. Soft Matter 6, 739–742 (2010).
Reinheimer, M. A., Mussati, S. & Scenna, N. J. Influence of product composition and operating conditions on the unsteady behavior of hard candy cooling process. J. Food Eng. 101, 409–416 (2010).
Lutolf, M. P. & Hubbell, J. A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nature Biotechnol. 23, 47–55 (2005).
Tibbitt, M. W. & Anseth, K. S. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol. Bioeng. 103, 655–663 (2009).
Heiber, J., Clemens, F., Graule, T. & Hülsenberg, D. Thermoplastic extrusion to highly-loaded thin green fibres containing Pb(Zr,Ti)O3 . Adv. Eng. Mater. 7, 404–408 (2005).
Miller, J. S. et al. Bioactive hydrogels made from step-growth derived PEG-peptide macromers. Biomaterials 31, 3736–3743 (2010).
Moon, J. J., Hahn, M. S., Kim, I., Nsiah, B. A. & West, J. L. Micropatterning of poly(ethylene glycol) diacrylate hydrogels with biomolecules to regulate and guide endothelial morphogenesis. Tissue Eng. Part A 15, 579–585 (2009).
DeLong, S. A., Moon, J. J. & West, J. L. Covalently immobilized gradients of bFGF on hydrogel scaffolds for directed cell migration. Biomaterials 26, 3227–3234 (2005).
Stefonek, T. J. & Masters, K. S. Immobilized gradients of epidermal growth factor promote accelerated and directed keratinocyte migration. Wound Repair Regen. 15, 847–855 (2007).
Leslie-Barbick, J. E., Moon, J. J. & West, J. L. Covalently-immobilized vascular endothelial growth factor promotes endothelial cell tubulogenesis in poly(ethylene glycol) diacrylate hydrogels. J. Biomater. Sci. Poly. Edn 20, 1763–1779 (2009).
Sundararaghavan, H. G., Monteiro, G. A., Firestein, B. L. & Shreiber, D. I. Neurite growth in 3D collagen gels with gradients of mechanical properties. Biotechnol. Bioeng. 102, 632–643 (2009).
Nemir, S., Hayenga, H. N. & West, J. L. PEGDA hydrogels with patterned elasticity: Novel tools for the study of cell response to substrate rigidity. Biotechnol. Bioeng. 105, 636–644 (2010).
Tsang, V. L. et al. Fabrication of 3D hepatic tissues by additive photopatterning of cellular hydrogels. FASEB J. 21, 790–801 (2007).
Thorsen, T., Maerkl, S. J. & Quake, S. R. Microfluidic large-scale integration. Science 298, 580–584 (2002).
Atencia, J. & Beebe, D. J. Controlled microfluidic interfaces. Nature 437, 648–655 (2005).
Acknowledgements
We thank the large number of open source and related projects that critically facilitated this work, including Arduino.cc, RepRap.org, MakerBot.com, Replicat.org, MakerGear.com, Ultimachine.com, Hive76.org, Python.org, Hugin.SourceForge.net, ImageMagick.org, Blender.org, Enblend.sourceforge.net, NIH ImageJ, and Fiji.sc. We thank R. J. Vlacich and C. D. Thompson for assistance with precision pneumatic extrusion, A. Dominguez for assistance with red blood cell isolation, and Y-J. Chen for assistance with transduction. This work was supported in part by grants from the US National Institutes of Health (EB00262, EB08396, GM74048), the Penn Center for Engineering Cells and Regeneration, and the American Heart Association-Jon Holden DeHaan Foundation. Individual fellowship support was provided by R. L. Kirschstein National Research Service Awards from NIH (J.S.M., HL099031; K.R.S., DK091007), the National Science Foundation IGERT program (M.T.Y., DGE-0221664), and the American Heart Association (X.Y., 10POST4220014).
Author information
Authors and Affiliations
Contributions
J.S.M. and C.S.C. conceived and initiated the project. J.S.M., K.R.S., M.T.Y., B.M.B., D-H.T.N., D.M.C., E.T., A.A.C., P.A.G., X.Y., and R.C. designed and performed experiments. C.S.C. and S.N.B. supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 780 kb)
Supplementary Movie 1
Supplementary Movie 1 (MP4 1806 kb)
Supplementary Movie 2
Supplementary Movie 2 (MP4 8809 kb)
Supplementary Movie 3
Supplementary Movie 3 (MP4 11313 kb)
Supplementary Movie 4
Supplementary Movie 4 (MP4 10156 kb)
Supplementary Movie 5
Supplementary Movie 5 (MP4 10355 kb)
Rights and permissions
About this article
Cite this article
Miller, J., Stevens, K., Yang, M. et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nature Mater 11, 768–774 (2012). https://doi.org/10.1038/nmat3357
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat3357
This article is cited by
-
Embedded macrophages induce intravascular coagulation in 3D blood vessel-on-chip
Biomedical Microdevices (2024)
-
Early introduction of 3D modeling modules promotes the development of simulation skills in downstream biomedical engineering curricula
Journal of Biological Engineering (2023)
-
Engineering of MSCs sheet for the prevention of myocardial ischemia and for left ventricle remodeling
Stem Cell Research & Therapy (2023)
-
Controlled tumor heterogeneity in a co-culture system by 3D bio-printed tumor-on-chip model
Scientific Reports (2023)
-
Large-scale perfused tissues via synthetic 3D soft microfluidics
Nature Communications (2023)