Abstract
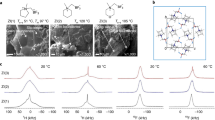
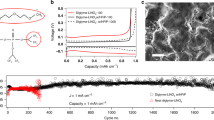
The future of lithium metal batteries as a widespread, safe and reliable form of high-energy-density rechargeable battery depends on a significant advancement in the electrolyte material used in these devices. Molecular solvent-based electrolytes have been superceded by polymer electrolytes in some prototype devices1, primarily in a drive to overcome leakage and flammability problems, but these often exhibit low ionic conductivity and prohibitively poor lithium-ion transport2,3,4. To overcome this, it is necessary to encourage dissociation of the lithium ion from the anionic polymer backbone, ideally without the introduction of competing, mobile ionic species. Here we demonstrate the effect of zwitterionic compounds, where the cationic and anionic charges are immobilized on the same molecule, as extremely effective lithium ion 'dissociation enhancers'. The zwitterion produces electrolyte materials with conductivities up to seven times larger than the pure polyelectrolyte gels, a phenomenon that appears to be common to a number of different copolymer and solvent systems.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Morris, R.S. & Dixon, B.G. J. Power Sources 119–121, 487–491 (2003).
Gray, F.M. Polymer Electrolytes (Royal Society of Chemistry, Cambridge, 1991).
Gray, F.M. Solid Polymer Electrolytes–Fundamentals and Technological Applications (VCH, New York, 1991).
Scrosati, B., Croce, F. & Persi, L. Impedance spectroscopy study of PEO-based nano-composite polymer electrolytes. J. Electrochem. Soc. 147, 1718–1721 (2000).
Sun, J., MacFarlane, D.R. & Forsyth, M. Lithium polyelectrolyte-ionic liquid systems. Solid State Ionics 147, 333–339 (2000).
Tiyapiboonchaiya, C., Pringle, J.M., MacFarlane, D.R., Forsyth, M. & Sun, J. Polyelectrolyte-in-ionic-liquid electrolytes. Macromol. Chem. Phys. 204, 2147–2154 (2003).
Yoshizawa, M., Hirao, M., Akita, K.I. & Ohno, H. Ion conduction in zwitterionic-type molten salts and their polymers. J. Mater. Chem. 11, 1057–1062 (2001).
Ohno, H., Yoshizawa, M. & Ogihara, W. A new type of polymer gel electrolyte: zwitterionic liquid/polar polymer mixture. Electrochimica Acta. 48, 2079–2083 (2003).
Pringle, J.M., Forsyth, C.M., Forsyth, M. & MacFarlane, D.R. The Zwitterion 1-butylimidazolium-3-(n-butanesulphonate) Acta Crystallogr. E59, 1759–1761 (2003).
Adebahr, J. et al. Ion transport in polymer electrolytes containing nanoparticulate TiO2: the influence of polymer morphology. Phys. Chem. Chem. Phys. 5, 720–725 (2003).
Grosberg, A.Y. & Khokhlov, A.R. Statistical Physics of Macromolecules (AIP, New York, 1994).
Osawa, F. Polyelectrolytes (Marcel Dekker, New York, 1971).
Travas-Sejdic Steiner, J.R., Desilvestro, J. & Pickering, P. Ion conductivity of novel polyelectrolyte gels for secondary lithium-ion polymer batteries. Electrochim. Acta 46, 1461–1466 (2001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Tiyapiboonchaiya, C., Pringle, J., Sun, J. et al. The zwitterion effect in high-conductivity polyelectrolyte materials. Nature Mater 3, 29–32 (2004). https://doi.org/10.1038/nmat1044
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat1044
This article is cited by
-
Force-induced ion generation in zwitterionic hydrogels for a sensitive silent-speech sensor
Nature Communications (2023)
-
Lean-water hydrogel electrolyte for zinc ion batteries
Nature Communications (2023)
-
Zwitterion-doped self-supporting single-ion conducting polymer electrolyte membrane for dendrite-free lithium metal secondary batteries
Science China Materials (2023)
-
Zwitterionic materials with disorder and plasticity and their application as non-volatile solid or liquid electrolytes
Nature Materials (2022)
-
Building Ultra-Stable and Low-Polarization Composite Zn Anode Interface via Hydrated Polyzwitterionic Electrolyte Construction
Nano-Micro Letters (2022)