Abstract
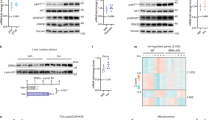
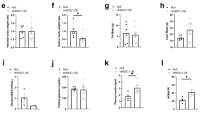
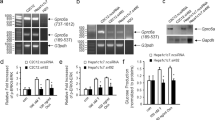
The transcription factor, signal transducer and activator of transcription-3 (STAT-3) contributes to various physiological processes. Here we show that mice with liver-specific deficiency in STAT-3, achieved using the Cre-loxP system, show insulin resistance associated with increased hepatic expression of gluconeogenic genes. Restoration of hepatic STAT-3 expression in these mice, using adenovirus-mediated gene transfer, corrected the metabolic abnormalities and the alterations in hepatic expression of gluconeogenic genes. Overexpression of STAT-3 in cultured hepatocytes inhibited gluconeogenic gene expression independently of peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α), an upstream regulator of gluconeogenic genes. Liver-specific expression of a constitutively active form of STAT-3, achieved by infection with an adenovirus vector, markedly reduced blood glucose, plasma insulin concentrations and hepatic gluconeogenic gene expression in diabetic mice. Hepatic STAT-3 signaling is thus essential for normal glucose homeostasis and may provide new therapeutic targets for diabetes mellitus.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Taylor, S.I. Deconstructing type 2 diabetes. Cell 97, 9–12 (1999).
Radziuk, J. & Pye, S. Hepatic glucose uptake, gluconeogenesis and the regulation of glycogen synthesis. Diabet. Metab. Res. Rev. 17, 250–272 (2001).
Shimomura, I. et al. Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice. Mol. Cell 6, 77–86 (2000).
Michael, M.D. et al. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol. Cell 6, 87–97 (2000).
Trinh, K.Y., O'Doherty, R.M., Anderson, P., Lange, A.J. & Newgard, C.B. Perturbation of fuel homeostasis caused by overexpression of the glucose-6-phosphatase catalytic subunit in liver of normal rats. J. Biol. Chem. 273, 31615–31620 (1998).
Valera, A., Pujol, A., Pelegrin, M. & Bosch, F. Transgenic mice overexpressing phosphoenolpyruvate carboxykinase develop non-insulin-dependent diabetes mellitus. Proc. Natl. Acad. Sci. USA 91, 9151–9154 (1994).
Rosella, G. et al. Impaired glucose tolerance and increased weight gain in transgenic rats overexpressing a non-insulin-responsive phosphoenolpyruvate carboxykinase gene. Mol. Endocrinol. 9, 1396–1404 (1995).
Sun, Y. et al. Phosphoenolpyruvate carboxykinase overexpression selectively attenuates insulin signaling and hepatic insulin sensitivity in transgenic mice. J. Biol. Chem. 277, 23301–23307 (2002).
O'Brien, R.M. & Granner, D.K. Regulation of gene expression by insulin. Physiol. Rev. 76, 1109–1161 (1996).
Christ, B., Yazici, E. & Nath, A. Phosphatidylinositol 3-kinase and protein kinase C contribute to the inhibition by interleukin 6 of phosphoenolpyruvate carboxykinase gene expression in cultured rat hepatocytes. Hepatology 31, 461–468 (2000).
Metzger, S. et al. Interleukin-6 secretion in mice is associated with reduced glucose-6-phosphatase and liver glycogen levels. Am. J. Physiol. 273, E262–E267 (1997).
Akira, S., Taga, T. & Kishimoto, T. Interleukin-6 in biology and medicine. Adv. Immunol. 54, 1–78 (1993).
Febbraio, M.A. & Pedersen, B.K. Muscle-derived interleukin-6: mechanisms for activation and possible biological roles. FASEB J. 16, 1335–1347 (2002).
Vgontzas, A.N., Papanicolaou, D.A., Bixler, E.O., Kales, A., Tyson, K. & Chrousos, G.P. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: role of sleep disturbance and obesity. J. Clin. Endocrinol. Metab. 82, 1313–1316 (1997).
Levy, D.E. & Lee, C.-K. What does Stat3 do? J. Clin. Invest. 109, 1143–1148 (2002).
Takeda, K. et al. Targeted disruption of the mouse Stat3 gene leads to early embryonic lethality. Proc. Natl. Acad. Sci. USA 94, 3801–3804 (1997).
Yaker, S. et al. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc. Natl. Acad. Sci. USA 96, 7324–7329 (1999).
Takeda, K. et al. Stat3 activation is responsible for IL-6-dependent T cell proliferation through preventing apoptosis: generation and characterization of T cell-specific Stat3-deficient mice. J. Immunol. 161, 4652–4560 (1998).
Yoon, J.C. et al. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature 413, 131–138 (2001).
Horton, J.D., Goldstein, J.L. & Brown, M.S. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Invest. 109, 1125–1131 (2002).
Miyake, K. et al. Hyperinsulinemia, glucose intolerance, and dyslipidemia induced by acute inhibition of phosphoinositide 3-kinase signaling in the liver. J. Clin. Invest. 110, 1483–1491 (2002).
Minami, M. et al. STAT3 activation is a critical step in gp130-mediated terminal differentiation and growth arrest of a myeloid cell line. Proc. Natl. Acad. Sci. USA 93, 3963–3966 (1996).
Nakae, J., Kitamura, T., Silver, D.L. & Accili, D. The forkhead transcription factor Foxo1 (Fkhr) confers insulin sensitivity. onto glucose-6-phosphatase expression. J. Clin. Invest. 108, 1359–67 (2001).
Puigserver, P et al. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1a interaction. Nature 423, 550–555. (2003).
Scott, D.K., O'Doherty, R.M., Stafford, J.M., Newgard, C.B. & Granner, D.K. Therepression of hormone-activated PEPCK gene expression by glucose is insulin-independent but requires glucose metabolism. J. Biol. Chem. 273, 24145–24151 (1998).
Kotani, K et al. Dominant negative forms of Akt (protein kinase B) and atypical protein kinase Cλ do not prevent insulin inhibition of phosphoenolpyruvate carboxykinase gene transcription. J. Biol. Chem. 274, 21305–21312 (1998).
Herzig, S et al. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature 413, 179–183 (2001).
Lee, G.-H. et al. Abnormal splicing of the leptin receptor in diabetic mice. Nature 379, 632–635 (1996).
Bromberg, J.F. et al. Stat3 as an oncogene. Cell 98, 295–303 (1999).
Strassmann, G., Fong, M., Windsor, S. & Neta, R. The role of interleukin-6 in lipopolysaccharide-induced weight loss, hypoglycemia and fibrinogen production in vivo. Cytokine 5, 285–290 (1993).
Wallenius, V. et al. Interleukin-6-deficient mice develop mature-onset obesity. Nature Med. 8, 75–79 (2002).
Bluher, M et al. Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance. Dev. Cell. 3, 25–38 (2002).
Kitamura, T. et al. Insulin-induced phosphorylation and activation of cyclic nucleotide phosphodiesterase 3B by the serine-threonine kinase Akt. Mol. Cell. Biol. 19, 6286–6296 (1999).
Ozaki, M. et al. Inhibition of the Rac1 GTPase protects against nonlethal ischemia/reperfusion-induced necrosis and apoptosis in vivo. FASEB J. 14, 418–429 (2000).
Ren, J.M., Marshall, B.A., Mueckler, M.M., McCaleb, M., Amatruda, J.M., & Shulman, G.I. Overexpression of Glut4 protein in muscle increases basal and insulin-stimulated whole body glucose disposal in conscious mice. J. Clin. Invest. 95, 429–432 (1995).
Matsumoto, M. et al. PKCλ in liver mediates insulin-induced SREBP-1c expression and determines both hepatic lipid content and overall insulin sensitivity. J. Clin. Invest. 112, 935–944 (2003).
Matsumoto, M. et al. Role of the insulin receptor substrate 1 and phosphatidylinositol 3-kinase signaling pathway in insulin-induced expression of sterol regulatory element binding protein 1c and glucokinase genes in rat hepatocytes. Diabetes 51, 1672–1680 (2002).
Acknowledgements
We thank J.E. Darnell Jr. for the STAT-3C-encoding cDNA, T. Noguchi for the Gck probe, H. Shimano for the Srebf1 probe, H. Nakajima for the G6pc probe, N. Iritani for the Fasn probe, D. Accili for the adenovirus encoding hemagglutinin-tagged, wild-type FOXO-1, and D.K. Granner for the HL1C cells. This work was supported by a grant from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (to M.K. and W.O.) and a grant from the Cooperative Link of Unique Science and Technology for Economy Revitalization (to M.K.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Inoue, H., Ogawa, W., Ozaki, M. et al. Role of STAT-3 in regulation of hepatic gluconeogenic genes and carbohydrate metabolism in vivo. Nat Med 10, 168–174 (2004). https://doi.org/10.1038/nm980
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm980
This article is cited by
-
Opportunities and challenges: interleukin-22 comprehensively regulates polycystic ovary syndrome from metabolic and immune aspects
Journal of Ovarian Research (2023)
-
The transcription factor ATF3 switches cell death from apoptosis to necroptosis in hepatic steatosis in male mice
Nature Communications (2023)
-
Pirfenidone ameliorates liver steatosis by targeting the STAT3-SCD1 axis
Inflammation Research (2023)
-
Signaling pathways in obesity: mechanisms and therapeutic interventions
Signal Transduction and Targeted Therapy (2022)
-
Differences in transcription regulation of diurnal metabolic support to physiologically contrasting seasonal life-history states in migratory songbirds
Journal of Ornithology (2022)