Abstract
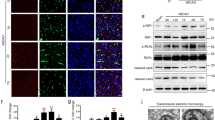
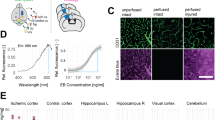
Thrombolytic treatment of ischemic stroke with tissue plasminogen activator (tPA) is markedly limited owing to concerns about hemorrhagic complications and the requirement that tPA be administered within 3 h of symptoms. Here we report that tPA activation of latent platelet-derived growth factor-CC (PDGF-CC) may explain these limitations. Intraventricular injection of tPA or active PDGF-CC, in the absence of ischemia, leads to significant increases in cerebrovascular permeability. In contrast, co-injection of neutralizing antibodies to PDGF-CC with tPA blocks this increased permeability, indicating that PDGF-CC is a downstream substrate of tPA within the neurovascular unit. These effects are mediated through activation of PDGF-α receptors (PDGFR-α) on perivascular astrocytes, and treatment of mice with the PDGFR-α antagonist imatinib after ischemic stroke reduces both cerebrovascular permeability and hemorrhagic complications associated with late administration of thrombolytic tPA. These data demonstrate that PDGF signaling regulates blood-brain barrier permeability and suggest potential new strategies for stroke treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Thom, T. et al. Heart disease and stroke statistics—2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 113, e85–e151 (2006).
Hou, S.T. & MacManus, J.P. Molecular mechanisms of cerebral ischemia-induced neuronal death. Int. Rev. Cytol. 221, 93–148 (2002).
Marler, J.R. & Goldstein, L.B. Medicine. Stroke—tPA and the clinic. Science 301, 1677 (2003).
Tsirka, S.E., Gualandris, A., Amaral, D.G. & Strickland, S. Excitotoxin-induced neuronal degeneration and seizure are mediated by tissue plasminogen activator. Nature 377, 340–344 (1995).
Tsirka, S.E., Rogove, A.D., Bugge, T.H., Degen, J.L. & Strickland, S. An extracellular proteolytic cascade promotes neuronal degeneration in the mouse hippocampus. J. Neurosci. 17, 543–552 (1997).
Wang, Y.F. et al. Tissue plasminogen activator (tPA) increases neuronal damage after focal cerebral ischemia in wild-type and tPA-deficient mice. Nat. Med. 4, 228–231 (1998).
Nagai, N., De Mol, M., Lijnen, H.R., Carmeliet, P. & Collen, D. Role of plasminogen system components in focal cerebral ischemic infarction: a gene targeting and gene transfer study in mice. Circulation 99, 2440–2444 (1999).
Yepes, M. et al. Neuroserpin reduces cerebral infarct volume and protects neurons from ischemia-induced apoptosis. Blood 96, 569–576 (2000).
Nicole, O. et al. The proteolytic activity of tissue-plasminogen activator enhances NMDA receptor-mediated signaling. Nat. Med. 7, 59–64 (2001).
Cinelli, P. et al. Neuroserpin, a neuroprotective factor in focal ischemic stroke. Mol. Cell. Neurosci. 18, 443–457 (2001).
Yepes, M. et al. Regulation of seizure spreading by neuroserpin and tissue-type plasminogen activator is plasminogen-independent. J. Clin. Invest. 109, 1571–1578 (2002).
Pawlak, R., Melchor, J.P., Matys, T., Skrzypiec, A.E. & Strickland, S. Ethanol-withdrawal seizures are controlled by tissue plasminogen activator via modulation of NR2B-containing NMDA receptors. Proc. Natl. Acad. Sci. USA 102, 443–448 (2005).
Tabrizi, P. et al. Tissue plasminogen activator (tPA) deficiency exacerbates cerebrovascular fibrin deposition and brain injury in a murine stroke model: studies in tPA-deficient mice and wild-type mice on a matched genetic background. Arterioscler. Thromb. Vasc. Biol. 19, 2801–2806 (1999).
Zivin, J.A., Fisher, M., DeGirolami, U., Hemenway, C.C. & Stashak, J.A. Tissue plasminogen activator reduces neurological damage after cerebral embolism. Science 230, 1289–1292 (1985).
The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N. Engl. J. Med. 333, 1581–1587 (1995).
Zhang, Z. et al. Adjuvant treatment with neuroserpin increases the therapeutic window for tissue-type plasminogen activator administration in a rat model of embolic stroke. Circulation 106, 740–745 (2002).
Yepes, M. et al. Tissue-type plasminogen activator induces opening of the blood-brain barrier via the LDL receptor–related protein. J. Clin. Invest. 112, 1533–1540 (2003).
Herz, J. LRP: a bright beacon at the blood-brain barrier. J. Clin. Invest. 112, 1483–1485 (2003).
Wang, X. et al. Lipoprotein receptor–mediated induction of matrix metalloproteinase by tissue plasminogen activator. Nat. Med. 9, 1313–1317 (2003).
Fredriksson, L., Li, H., Fieber, C., Li, X. & Eriksson, U. Tissue plasminogen activator is a potent activator of PDGF-CC. EMBO J. 23, 3793–3802 (2004).
Li, X. et al. PDGF-C is a new protease-activated ligand for the PDGF-α receptor. Nat. Cell Biol. 2, 302–309 (2000).
Fredriksson, L., Ehnman, M., Fieber, C. & Eriksson, U. Structural requirements for activation of latent platelet-derived growth factor CC by tissue plasminogen activator. J. Biol. Chem. 280, 26856–26862 (2005).
Hart, C.E. et al. Two classes of PDGF receptor recognize different isoforms of PDGF. Science 240, 1529–1531 (1988).
Polavarapu, R. et al. Tissue-type plasminogen activator–mediated shedding of astrocytic low-density lipoprotein receptor–related protein increases the permeability of the neurovascular unit. Blood 109, 3270–3278 (2007).
Willnow, T.E. & Herz, J. Genetic deficiency in low density lipoprotein receptor–related protein confers cellular resistance to Pseudomonas exotoxin A. Evidence that this protein is required for uptake and degradation of multiple ligands. J. Cell Sci. 107, 719–726 (1994).
Yu, H. et al. Control elements between −9.5 and −3.0 kb in the human tissue-type plasminogen activator gene promoter direct spatial and inducible expression to the murine brain. Eur. J. Neurosci. 14, 799–808 (2001).
Hamilton, T.G., Klinghoffer, R.A., Corrin, P.D. & Soriano, P. Evolutionary divergence of platelet-derived growth factor α receptor signaling mechanisms. Mol. Cell. Biol. 23, 4013–4025 (2003).
Ding, H. et al. A specific requirement for PDGF-C in palate formation and PDGFR-α signaling. Nat. Genet. 36, 1111–1116 (2004).
Wolf, B.B., Lopes, M.B., VandenBerg, S.R. & Gonias, S.L. Characterization and immunohistochemical localization of α 2-macroglobulin receptor (low-density lipoprotein receptor–related protein) in human brain. Am. J. Pathol. 141, 37–42 (1992).
Nagai, N. et al. Tissue-type plasminogen activator has paradoxical roles in focal cerebral ischemic injury by thrombotic middle cerebral artery occlusion with mild or severe photochemical damage in mice. J. Cereb. Blood Flow Metab. 22, 648–651 (2002).
Nagai, N., Suzuki, Y., Van, H.B., Lijnen, H.R. & Collen, D. Effects of plasminogen activator inhibitor-1 on ischemic brain injury in permanent and thrombotic middle cerebral artery occlusion models in mice. J. Thromb. Haemost. 3, 1379–1384 (2005).
Capdeville, R., Buchdunger, E., Zimmermann, J. & Matter, A. Glivec (STI571, imatinib), a rationally developed, targeted anticancer drug. Nat. Rev. Drug Discov. 1, 493–502 (2002).
The National Institute of Neurological Disorders and Stroke t-PA Stroke Study Group. Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke. Stroke 28, 2109–2118 (1997).
Larrue, V., von Kummer, R.R., Muller, A. & Bluhmki, E. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European-Australasian Acute Stroke Study (ECASS II). Stroke 32, 438–441 (2001).
Thomalla, G. et al. Two tales: hemorrhagic transformation but not parenchymal hemorrhage after thrombolysis is related to severity and duration of ischemia: MRI study of acute stroke patients treated with intravenous tissue plasminogen activator within 6 hours. Stroke 38, 313–318 (2007).
Vora, N.A. et al. Factors predicting hemorrhagic complications after multimodal reperfusion therapy for acute ischemic stroke. AJNR Am. J. Neuroradiol. 28, 1391–1394 (2007).
Yepes, M. & Lawrence, D.A. New functions for an old enzyme: nonhemostatic roles for tissue-type plasminogen activator in the central nervous system. Exp. Biol. Med. (Maywood) 229, 1097–1104 (2004).
Yepes, M. & Lawrence, D.A. Tissue-type plasminogen activator and neuroserpin: a well balanced act in the nervous system? Trends Cardiovasc. Med. 14, 173–180 (2004).
Zhuo, M. et al. Role of tissue plasminogen activator receptor LRP in hippocampal long- term potentiation. J. Neurosci. 20, 542–549 (2000).
Hao, Z. et al. New transgenic evidence for a system of sympathetic axons able to express tissue plasminogen activator (t-PA) within arterial/arteriolar walls. Blood 108, 200–202 (2006).
Gualandris, A., Jones, T.E., Strickland, S. & Tsirka, S.E. Membrane depolarization induces calcium-dependent secretion of tissue plasminogen activator. J. Neurosci. 16, 2220–2225 (1996).
Breedveld, P. et al. The effect of Bcrp1 (Abcg2) on the in vivo pharmacokinetics and brain penetration of imatinib mesylate (Gleevec): implications for the use of breast cancer resistance protein and P-glycoprotein inhibitors to enable the brain penetration of imatinib in patients. Cancer Res. 65, 2577–2582 (2005).
Melchor, J.P. & Strickland, S. Tissue plasminogen activator in central nervous system physiology and pathology. Thromb. Haemost. 93, 655–660 (2005).
Ponten, A. et al. Transgenic overexpression of platelet-derived growth factor-C in the mouse heart induces cardiac fibrosis, hypertrophy, and dilated cardiomyopathy. Am. J. Pathol. 163, 673–682 (2003).
McMahon, G.A. et al. Plasminogen activator inhibitor-1 regulates tumor growth and angiogenesis. J. Biol. Chem. 276, 33964–33968 (2001).
Acknowledgements
We want to thank P. Soriano (Fred Hutchinson Cancer Research Center) for the PDGFR-α–GFP mice; A. Nagy (Samuel Lunenfeld Research Institute, Mount Sinai Hospital) for the PDGF-C–knockout mice; M. Wang, G. Schielke and D. Lombardi for helpful discussions and critical reading of the manuscript; N. Gorlatova for surface plasmon resonance analysis; and S. Rezaian and M. Wahl for technical assistance. This work was supported by National Institutes of Health grants HL55374, HL55747, HL54710 and HL57346 (to D.A.L.); HL50784 and HL54710 (to D.K.S.); NS49478 (to M.Y.); and grants from Karolinska Institutet, Novo Nordisk Foundation, Swedish Research Council, Swedish Cancer Foundation, the LeDucq Foundation and IngaBritt and Arne Lundberg Foundation (to U.E. and C.B.).
Author information
Authors and Affiliations
Contributions
E.J.S. and L.F. conceived, designed and performed the research and wrote the paper, M.G., E.F., J.C., J.A., Y.G., K.P. and K.M. performed the research, M.Y. conceived the research, D.K.S. and C.B. designed the research and contributed vital reagents, U.E. and D.A.L. conceived and designed the research and wrote the paper.
Corresponding authors
Supplementary information
Supplementary Text and Figures
Supplementary Figs. 1–6 and Supplementary Methods 1 and 2 (PDF 1253 kb)
Rights and permissions
About this article
Cite this article
Su, E., Fredriksson, L., Geyer, M. et al. Activation of PDGF-CC by tissue plasminogen activator impairs blood-brain barrier integrity during ischemic stroke. Nat Med 14, 731–737 (2008). https://doi.org/10.1038/nm1787
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1787
This article is cited by
-
Effects of acetazolamide combined with remote ischemic preconditioning on risk of acute mountain sickness: a randomized clinical trial
BMC Medicine (2024)
-
Egr-1 is a key regulator of the blood-brain barrier damage induced by meningitic Escherichia coli
Cell Communication and Signaling (2024)
-
Tranexamic acid for haemostasis and beyond: does dose matter?
Thrombosis Journal (2023)
-
Hemorrhagic Conversion of Acute Ischemic Stroke
Neurotherapeutics (2023)
-
Imatinib attenuates reperfusion injury in a rat model of acute myocardial infarction
Basic Research in Cardiology (2023)