Abstract
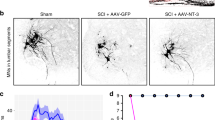
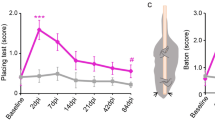
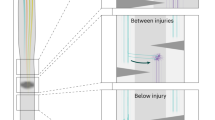
Spinal cord injuries (SCIs) in humans1,2 and experimental animals3,4,5,6 are often associated with varying degrees of spontaneous functional recovery during the first months after injury. Such recovery is widely attributed to axons spared from injury that descend from the brain and bypass incomplete lesions, but its mechanisms are uncertain. To investigate the neural basis of spontaneous recovery, we used kinematic, physiological and anatomical analyses to evaluate mice with various combinations of spatially and temporally separated lateral hemisections with or without the excitotoxic ablation of intrinsic spinal cord neurons. We show that propriospinal relay connections that bypass one or more injury sites are able to mediate spontaneous functional recovery and supraspinal control of stepping, even when there has been essentially total and irreversible interruption of long descending supraspinal pathways in mice. Our findings show that pronounced functional recovery can occur after severe SCI without the maintenance or regeneration of direct projections from the brain past the lesion and can be mediated by the reorganization of descending and propriospinal connections4,7,8,9. Targeting interventions toward augmenting the remodeling of relay connections may provide new therapeutic strategies to bypass lesions and restore function after SCI and in other conditions such as stroke and multiple sclerosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Fawcett, J.W. et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord 45, 190–205 (2007).
Dobkin, B. et al. The evolution of walking-related outcomes over the first 12 weeks of rehabilitation for incomplete traumatic spinal cord injury: the multicenter randomized spinal cord injury locomotor trial. Neurorehabil. Neural Repair 21, 25–35 (2007).
Weidner, N., Ner, A., Salimi, N. & Tuszynski, M. Spontaneous corticospinal axonal plasticity and functional recovery after adult central nervous system injury. Proc. Natl. Acad. Sci. USA 98, 3513–3518 (2001).
Bareyre, F.M. et al. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat. Neurosci. 7, 269–277 (2004).
Courtine, G. et al. Performance of locomotion and foot grasping following a unilateral thoracic corticospinal tract lesion in monkeys (Macaca mulatta). Brain 128, 2338–2358 (2005).
Ballermann, M. & Fouad, K. Spontaneous locomotor recovery in spinal cord injured rats is accompanied by anatomical plasticity of reticulospinal fibers. Eur. J. Neurosci. 23, 1988–1996 (2006).
Raineteau, O. & Schwab, M.E. Plasticity of motor systems after incomplete spinal cord injury. Nat. Rev. Neurosci. 2, 263–273 (2001).
Kerschensteiner, M. et al. Remodeling of axonal connections contributes to recovery in an animal model of multiple sclerosis. J. Exp. Med. 200, 1027–1038 (2004).
Jankowska, E. & Edgley, S.A. How can corticospinal tract neurons contribute to ipsilateral movements? A question with implications for recovery of motor functions. Neuroscientist 12, 67–79 (2006).
Jane, J.A., Evans, J.P. & Fisher, L.E. An investigation concerning the restitution of motor function following injury to the spinal cord. J. Neurosurg. 21, 167–171 (1964).
Basbaum, A.I. Conduction of the effects of noxious stimulation by short-fiber multisynaptic systems of the spinal cord in the rat. Exp. Neurol. 40, 699–716 (1973).
Stelzner, D.J. & Cullen, J.M. Do propriospinal projections contribute to hindlimb recovery when all long tracts are cut in neonatal or weanling rats? Exp. Neurol. 114, 193–205 (1991).
Duffy, M.T., Simpson, S.B.J., Liebich, D.R. & Davis, B.M. Origin of spinal cord axons in the lizard regenerated tail: supernormal projections from local spinal neurons. J. Comp. Neurol. 293, 208–222 (1990).
Nantwi, K.D., El-Bohy, A.A., Schrimsher, G.W., Reier, P.J. & Goshgarian, H.G. Spontaneous recovery in a paralyzed hemidiaphragm following upper cervical spinal cord injury in adult rats. Neurorehabil. Neural Repair 13, 225–234 (1999).
Kiehn, O. Locomotor circuits in the mammalian spinal cord. Annu. Rev. Neurosci. 29, 279–306 (2006).
Leblond, H., L'Esperance, M., Orsal, D. & Rossignol, S. Treadmill locomotion in the intact and spinal mouse. J. Neurosci. 23, 11411–11419 (2003).
Sofroniew, M.V., Galletly, N.P., Isacson, O. & Svendsen, C.N. Survival of adult basal forebrain cholinergic neurons after loss of target neurons. Science 247, 338–342 (1990).
Magnuson, D.S. et al. Comparing deficits following excitotoxic and contusion injuries in the thoracic and lumbar spinal cord of the adult rat. Exp. Neurol. 156, 191–204 (1999).
Fong, A.J. et al. Spinal cord–transected mice learn to step in response to quipazine treatment and robotic training. J. Neurosci. 25, 11738–11747 (2005).
Edgerton, V.R. et al. Retraining the injured spinal cord. J. Physiol. (Lond.) 533, 15–22 (2001).
Zaporozhets, E., Cowley, K.C. & Schmidt, B.J. Propriospinal neurons contribute to bulbospinal transmission of the locomotor command signal in the neonatal rat spinal cord. J. Physiol. (Lond.) 572, 443–458 (2006).
Courtine, G. et al. Can experiments in nonhuman primates expedite the translation of treatments for spinal cord injury in humans? Nat. Med. 13, 561–566 (2007).
Schwab, M.E. Repairing the injured spinal cord. Science 295, 1029–1031 (2002).
Filbin, M.T. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat. Rev. Neurosci. 4, 703–713 (2003).
Silver, J. & Miller, J.H. Regeneration beyond the glial scar. Nat. Rev. Neurosci. 5, 146–156 (2004).
Irwin, N., Li, Y.M., O'Toole, J.E. & Benowitz, L.I. Mst3b, a purine-sensitive Ste20-like protein kinase, regulates axon outgrowth. Proc. Natl. Acad. Sci. USA 103, 18320–18325 (2006).
Thuret, S., Moon, L.D. & Gage, F.H. Therapeutic interventions after spinal cord injury. Nat. Rev. Neurosci. 7, 628–643 (2006).
Faulkner, J.R. et al. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J. Neurosci. 24, 2143–2155 (2004).
Courtine, G. et al. Kinematic and EMG determinants in quadrupedal locomotion of a non-human primate (Rhesus). J. Neurophysiol. 93, 3127–3145 (2005).
Recktenwald, M.R. et al. Effects of spaceflight on rhesus quadrupedal locomotion after return to 1G. J. Neurophysiol. 81, 2451–2463 (1999).
Acknowledgements
This work was supported by grants from the National Institutes of Health (NS16333), the Christopher and Dana Reeve Foundation, the Adelson Medical Foundation and the Roman Reed Spinal Cord Injury Research Fund of California.
Author information
Authors and Affiliations
Contributions
G.C., R.R.R., V.R.E. and M.V.S. designed the experiments; G.C., B.S., R.R.R., H.Z., J.E.H., Y.A. and J.Q. performed the experiments; G.C., B.S., R.R.R., V.R.E. and M.V.S. analyzed the data and G.C., R.R.R., V.R.E. and M.V.S. wrote the paper.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figs. 1–5 (PDF 1512 kb)
Supplementary Video 1
Loss and recovery of supraspinal control of stepping after T12 hemisection. (MOV 72562 kb)
Supplementary Video 2
Delayed (10 wks) contralateral T12 hemisection abolishes recovery of supraspinal control of stepping. (MOV 6097 kb)
Supplementary Video 3a
Permanent lost of stepping after simultaneous hemisections at T12 and T7. (MOV 13026 kb)
Supplementary Video 3b
Loss and recovery of supraspinal control of stepping after T12 and delayed T7 hemisections. (MOV 12859 kb)
Supplementary Video 3c
Delayed complete T7 spinal transection abolishes recovered stepping after T12 hemisection. (MOV 9089 kb)
Supplementary Video 4a
T8- T10 NMDA lesion does not alter stepping in the absence of other injuries. (MOV 15108 kb)
Supplementary Video 4b
T8-T10 NMDA lesion abolishes recovered supraspinal control of stepping after T12 hemisection. (MOV 10986 kb)
Supplementary Video 4c
T8-T10 NMDA lesion abolishes recovered supraspinal control of bilateral stepping after two temporally separated unilateral hemisections at T12 (left) and T7 (right). (MOV 9475 kb)
Rights and permissions
About this article
Cite this article
Courtine, G., Song, B., Roy, R. et al. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat Med 14, 69–74 (2008). https://doi.org/10.1038/nm1682
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1682
This article is cited by
-
Functional plasticity of glutamatergic neurons of medullary reticular nuclei after spinal cord injury in mice
Nature Communications (2024)
-
Autonomic dysreflexia in urological practice: pathophysiology, prevention and treatment considerations
World Journal of Urology (2024)
-
Neural stem/progenitor cells from adult canine cervical spinal cord have the potential to differentiate into neural lineage cells
BMC Veterinary Research (2023)
-
Beyond treatment of chronic pain: a scoping review about epidural electrical spinal cord stimulation to restore sensorimotor and autonomic function after spinal cord injury
Neurological Research and Practice (2023)
-
Low oral dose of 4-methylumbelliferone reduces glial scar but is insufficient to induce functional recovery after spinal cord injury
Scientific Reports (2023)