Abstract
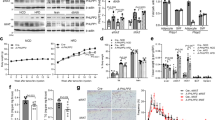
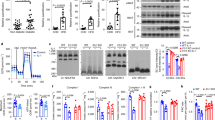
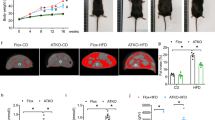
Adiponectin plays a central role as an antidiabetic and antiatherogenic adipokine. AdipoR1 and AdipoR2 serve as receptors for adiponectin in vitro, and their reduction in obesity seems to be correlated with reduced adiponectin sensitivity. Here we show that adenovirus-mediated expression of AdipoR1 and R2 in the liver of Lepr−/− mice increased AMP-activated protein kinase (AMPK) activation and peroxisome proliferator-activated receptor (PPAR)-α signaling pathways, respectively. Activation of AMPK reduced gluconeogenesis, whereas expression of the receptors in both cases increased fatty acid oxidation and lead to an amelioration of diabetes. Alternatively, targeted disruption of AdipoR1 resulted in the abrogation of adiponectin-induced AMPK activation, whereas that of AdipoR2 resulted in decreased activity of PPAR-α signaling pathways. Simultaneous disruption of both AdipoR1 and R2 abolished adiponectin binding and actions, resulting in increased tissue triglyceride content, inflammation and oxidative stress, and thus leading to insulin resistance and marked glucose intolerance. Therefore, AdipoR1 and R2 serve as the predominant receptors for adiponectin in vivo and play important roles in the regulation of glucose and lipid metabolism, inflammation and oxidative stress in vivo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hug, C. & Lodish, H.F. The role of the adipocyte hormone adiponectin in cardiovascular disease. Curr. Opin. Pharmacol. 5, 129–134 (2005).
Scherer, P.E. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes 55, 1537–1545 (2006).
Matsuzawa, Y. The metabolic syndrome and adipocytokines. FEBS Lett. 580, 2917–2921 (2006).
Kadowaki, T. et al. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Invest. 116, 1784–1792 (2006).
Fruebis, J. et al. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc. Natl. Acad. Sci. USA 98, 2005–2010 (2001).
Yamauchi, T. et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 7, 941–946 (2001).
Berg, A.H., Combs, T.P., Du, X., Brownlee, M. & Scherer, P.E. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat. Med. 7, 947–953 (2001).
Yamauchi, T. et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 8, 1288–1295 (2002).
Tomas, E. et al. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc. Natl. Acad. Sci. USA 99, 16309–16313 (2002).
Kersten, S., Desvergne, B. & Wahli, W. Roles of PPARs in health and disease. Nature 405, 421–424 (2000).
Yamauchi, T. et al. Globular adiponectin protected ob/ob mice from diabetes and apoE deficient mice from atherosclerosis. J. Biol. Chem. 278, 2461–2468 (2003).
Yamauchi, T. et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 423, 762–769 (2003).
Tsuchida, A. et al. Insulin/Foxo1 pathway regulates expression levels of adiponectin receptors and adiponectin sensitivity. J. Biol. Chem. 279, 30817–30822 (2004).
Wellen, K.E. & Hotamisligil, G.S. Inflammation, stress, and diabetes. J. Clin. Invest. 115, 1111–1119 (2005).
Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 414, 813–820 (2001).
Kubota, N. et al. Disruption of adiponectin causes insulin resistance and neointimal formation. J. Biol. Chem. 277, 25863–25866 (2002).
Maeda, N. et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat. Med. 8, 731–737 (2002).
Kubota, N. et al. Pioglitazone ameliorates insulin resistance and diabetes by both adiponectin-dependent and -independent pathways. J. Biol. Chem. 281, 8748–8755 (2006).
Nawrocki, A.R. et al. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J. Biol. Chem. 281, 2654–2660 (2006).
Miyake, K. et al. Hyperinsulinemia, glucose intolerance, and dyslipidemia induced by acute inhibition of phosphoinositide 3-kinase signaling in the liver. J. Clin. Invest. 110, 1483–1491 (2002).
Lochhead, P.A., Salt, I.P., Walker, K.S., Hardie, D.G. & Sutherland, C. 5-aminoimidazole-4-carboxamide riboside mimics the effects of insulin on the expression of the 2 key gluconeogenic genes PEPCK and glucose-6-phosphatase. Diabetes 49, 896–903 (2000).
Woods, A. et al. Characterization of the role of AMP-activated protein kinase in the regulation of glucose-activated gene expression using constitutively active and dominant negative forms of the kinase. Mol. Cell. Biol. 20, 6704–6711 (2000).
Matschinsky, F.M. et al. The network of glucokinase-expressing cells in glucose homeostasis and the potential of glucokinase activators for diabetes therapy. Diabetes 55, 1–12 (2006).
Long, Y.C. & Zierath, J.R. AMP-activated protein kinase signaling in metabolic regulation. J. Clin. Invest. 116, 1776–1783 (2006).
Shulman, G.I. Cellular mechanisms of insulin resistance. J. Clin. Invest. 106, 171–176 (2000).
Hotamisligil, G.S., Shargill, N.S. & Spiegelman, B.M. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 259, 87–91 (1993).
Weisberg, S.P. et al. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112, 1796–1808 (2003).
Xu, H. et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 112, 1821–1830 (2003).
Kanda, H. et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Invest. 116, 1494–1505 (2006).
Kamei, N. et al. Overexpression of MCP-1 in adipose tissues causes macrophage recruitment and insulin resistance. J. Biol. Chem. 281, 26602–26614 (2006).
Staels, B. et al. Activation of human aortic smooth-muscle cells is inhibited by PPARα but not by PPARγ activators. Nature 393, 790–793 (1998).
Delerive, P. et al. Peroxisome proliferator-activated receptor α negatively regulates the vascular inflammatory gene response by negative cross-talk with transcription factors NF-κB and AP-1. J. Biol. Chem. 274, 32048–32054 (1999).
Tsuchida, A. et al. Peroxisome proliferator-activated receptor (PPAR)α activation increases adiponectin receptors and reduces obesity-related inflammation in adipose tissue: comparison of activation of PPARα, PPARγ and their combination. Diabetes 54, 3358–3370 (2005).
Maddux, B.A. et al. Protection against oxidative stress-induced insulin resistance in rat L6 muscle cells by micromolar concentrations of α-lipoic acid. Diabetes 50, 404–410 (2001).
Rudich, A. et al. Prolonged oxidative stress impairs insulin-induced GLUT4 translocation in 3T3–L1 adipocytes. Diabetes 47, 1562–1569 (1998).
Toyama, T. et al. PPARalpha ligands activate antioxidant enzymes and suppress hepatic fibrosis in rats. Biochem. Biophys. Res. Commun. 324, 697–704 (2004).
Inoue, I. et al. The ligands/activators for peroxisome proliferator-activated receptor alpha (PPARalpha) and PPARgamma increase Cu2+,Zn2+-superoxide dismutase and decrease p22phox message expressions in primary endothelial cells. Metabolism 50, 3–11 (2001).
Cohen, P. et al. Role for stearoyl-CoA desaturase-1 in leptin-mediated weight loss. Science 297, 240–243 (2002).
Furukawa, S. et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest. 114, 1752–1761 (2004).
Hug, C. et al. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc. Natl. Acad. Sci. USA 101, 10308–10313 (2004).
Acknowledgements
We thank K. Kangawa, M. Yanagisawa, K. Nakao, M. Kasuga, T. Shimizu, T. Yokomizo, W. Ogawa, H. Watada, Y. Terauchi, I. Manabe, M. Yamaguchi, K. Kobayashi and Y. Iwata for advice and discussions. We are grateful to K. Okano, A. Itoh and K. Miyata for technical assistance. This work was supported by a grant from the Program for Promotion of Fundamental Studies in Health Sciences of the Organization for Pharmaceutical Safety and Research of Japan (to T.K.), a grant-in-aid for the Development of Innovative Technology from the Ministry of Education, Culture, Sports, Science and Technology (to T. K.) and Health Science Research Grants (Research on Human Genome and Gene Therapy) from the Ministry of Health, Labour and Welfare (to T. K. and T.Y.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Adenovirus-mediated expression of AdipoR1 or AdipoR2 in the liver of db/db mice. (PDF 303 kb)
Supplementary Fig. 2
2 Generation of AdipoR1 knockout, AdipoR2 knockout and AdipoR1˙R2 double knockout mice. (PDF 330 kb)
Supplementary Fig. 3
Plasma adiponectin and expression levels of AdipoR1 and AdipoR2 in the liver, skeletal muscle, and white adipose tissue from AdipoR1 knockout, AdipoR2 knockout and AdipoR1˙R2 double knockout mice. (PDF 219 kb)
Supplementary Fig. 4
Adiponectin action, glucose metabolism and insulin signal transduction in AdipoR1 knockout, AdipoR2 knockout and AdipoR1˙R2 double knockout mice. (PDF 284 kb)
Supplementary Fig. 5
Phosphorylation of AMPK stimulated with adiponectin and Akt stimulated with insulin and expression levels of molecules involved in lipid metabolism, inflammation and oxidative stress in skeletal muscle and white adipose tissue of AdipoR1 knockout, AdipoR2 knockout and AdipoR1˙R2 double knockout mice. (PDF 565 kb)
Rights and permissions
About this article
Cite this article
Yamauchi, T., Nio, Y., Maki, T. et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med 13, 332–339 (2007). https://doi.org/10.1038/nm1557
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1557
This article is cited by
-
Small-molecule agonist AdipoRon alleviates diabetic retinopathy through the AdipoR1/AMPK/EGR4 pathway
Journal of Translational Medicine (2024)
-
Running exercise alleviates hippocampal neuroinflammation and shifts the balance of microglial M1/M2 polarization through adiponectin/AdipoR1 pathway activation in mice exposed to chronic unpredictable stress
Molecular Psychiatry (2024)
-
The role of bidirectional communication between the adipokines and the endogenous opioid system in an experimental mouse model of colitis-associated colorectal cancer
Pharmacological Reports (2024)
-
Adiponectin Receptor Agonist Effectively Suppresses Hepatocellular Carcinoma Growth
Cell Biochemistry and Biophysics (2024)
-
Sex differences in obesity-induced renal lipid accumulation revealed by lipidomics: a role of adiponectin/AMPK axis
Biology of Sex Differences (2023)