Abstract
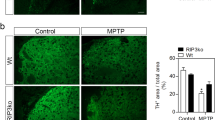
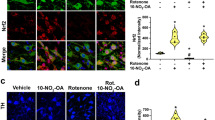
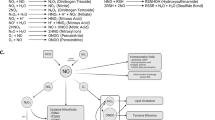
MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) damages dopaminergic neurons as seen in Parkinson disease. Here we show that after administration of MPTP to mice, there was a robust gliosis in the substantia nigra pars compacta associated with significant upregulation of inducible nitric oxide synthase (iNOS). These changes preceded or paralleled MPTP-induced dopaminergic neurodegeneration. We also show that mutant mice lacking the iNOS gene were significantly more resistant to MPTP than their wild-type littermates. This study demonstrates that iNOS is important in the MPTP neurotoxic process and indicates that inhibitors of iNOS may provide protective benefit in the treatment of Parkinson disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Fahn, S. in Cecil's Textbook of Medicine vol.18 (eds. Wyngaarden, J.B. & Smith, L.H.Jr.) 2143–2147 (Saunders, Philadelphia, 1988).
Pakkenberg, B., Moller, A., Gundersen, H.J.G., Mouritzen, A. & Pakkenberg, H. The absolute number of nerve cells in substantia nigra in normal subjects and in patients with Parkinson's disease estimated with an unbiased stereological method. J. Neurol. Neurosurg. Psychiat. 54, 30–33 (1991).
Hornykiewicz, O. & Kish, S.J. in Parkinson's Ddisease (eds. Yahr, M. & Bergmann, K.J.) 19– 34 (Raven, New York, 1987).
Kostic, V., Przedborski, S., Flaster, E. & Sternic, N. Early development of levodopa-induced dyskinesias and response fluctuations in young-onset Parkinson's disease. Neurology 41, 202–205 (1991).
Langston, J.W. & Irwin, I. MPTP: current concepts and controversies. Clin. Neuropharmacol. 9, 485– 507 (1986).
Przedborski, S. & Jackson-Lewis, V. Mechanisms of MPTP toxicity. Mov. Disord. 13, 35– 38 (1998).
Przedborski, S. & Jackson-Lewis, V. Experimental developments in movement disorders: update on proposed free radical mechanisms. Curr. Opin. Neurol 11, 335– 339 (1998).
Dawson, T.M. & Dawson, V.L. Nitric oxide synthase: role as a transmitter/mediator in the brain and endocrine system. Annu. Rev. Med. 47, 219–227 (1996).
Bredt, D.S., Glatt, C.E., Huang, P.L., Fotuhi, M., Dawson, T.M. & Snyder, S.H. Nitric oxide synthase protein and mRNA are discretely localized in neuronal populations of the mammalian CNS together with NADPH diaphorase. Neuron 7, 615–624 (1991).
Huang, P.L., Dawson, T.M., Bredt, D.S., Snyder, S.H. & Fishman, M.C. Targeted disruption of the neuronal nitric oxide synthase gene. Cell 75, 1273 –1285 (1993).
Lowenstein, C.J., Glatt, C.S., Bredt, D.S. & Snyder, S.H. Cloned and expressed macrophage nitric oxide synthase contrasts with the brain enzyme. Proc. Natl. Acad. Sci. USA 89, 6711– 6715 (1992).
Keilhoff, G. et al. Patterns of nitric oxide synthase at the messenger RNA and protein levels during early rat brain development. Neuroscience 75, 1193–1201 (1996).
Simmons, M.L. & Murphy, S. Induction of nitric oxide synthase in glial cells. J. Neurochem. 59, 897– 905 (1992).
Wallace, M.N. & Fredens, K. Activated astrocytes of the mouse hippocampus contain high levels of NADPH-diaphorase. Neuroreport 3, 953–956 (1992).
Iadecola, C., Xu, X., Zhang, F., el-Fakahany, E.E. & Ross, M.E. Marked induction of calcium-independent nitric oxide synthase activity after focal cerebral ischemia. J. Cereb. Blood Flow Metab. 15, 52–59 (1995).
Hara, H. et al. Brain distribution of nitric oxide synthase in neuronal or endothelial nitric oxide synthase mutant mice using [3H]L-NG-nitro-arginine autoradiography. Neuroscience 75, 881–890 (1996).
Dinerman, J.L., Dawson, T.M., Schell, M.J., Snowman, A. & Snyder, S.H. Endothelial nitric oxide synthase localized to hippocampal pyramidal cells: implications for synaptic plasticity. Proc. Natl. Acad. Sci. USA 91, 4214– 4218 (1994).
Dawson, T.M. & Dawson, V.L. in Nitric oxide. Sources and Detection of NO; NO Synthase (ed. Packer, L.) 349–358 (Academic, New York, 1996).
Schulz, J.B., Matthews, R.T., Muqit, M.M.K., Browne, S.E. & Beal, M.F. Inhibition of neuronal nitric oxide synthase by 7-nitroindazole protects against MPTP-induced neurotoxicity in mice. J. Neurochem. 64, 936– 939 (1995).
Przedborski, S. et al. Role of neuronal nitric oxide in MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine)-induced dopaminergic neurotoxicity. Proc. Natl. Acad. Sci. USA 93, 4565–4571 (1996).
Hantraye, P. et al. Inhibition of neuronal nitric oxide synthase prevents MPTP- induced parkinsonism in baboons. Nature Med. 2, 1017–1021 (1996).
Matthews, R.T., Yang, L.C. & Beal, M.F. S-methylthiocitrulline, a neuronal nitric oxide synthase inhibitor, protects against malonate and MPTP neurotoxicity. Exp. Neurol. 143, 282–286 (1997).
Hunot, S. et al. Nitric oxide synthase and neuronal vulnerability in Parkinson's disease. Neuroscience 72, 355– 363 (1996).
Adamson, D.C. et al. Immunologic NO synthase: elevation in severe AIDS dementia and induction by HIV-1 gp41. Science 274, 1917–1921 (1996).
Nathan, C. & Xie, Q.W. Regulation of biosynthesis of nitric oxide. J. Biol. Chem. 269, 13725– 13728 (1994).
Giovanni, A., Sieber, B.A., Heikkila, R.E. & Sonsalla, P.K. Correlation between the neostriatal content of the 1-methyl-4-phenylpyridinium species and dopaminergic neurotoxicity following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine administration to several strains of mice. J. Pharmacol. Exp. Ther. 257, 691–697 (1991).
Forno, L.S., DeLanney, L.E., Irwin, I., Di Monte, D. & Langston, J.W. Astrocytes and Parkinson's disease. Prog. Brain Res. 94, 429– 436 (1992).
Hirsch, E.C., Hunot, S., Damier, P. & Faucheux, B. Glial cells and inflammation in Parkinsons' disease: a role in neurodegeneration? Ann. Neurol. 44 (Suppl. 1), S115–S120 (1998).
Ridet, J.L., Malhotra, S.K., Privat, A. & Gage, F.H. Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci. 20, 570–577 (1997).
Gonzalez-Scarano, F. & Baltuch, G. Microglia as mediators of inflammatory and degenerative diseases. Annu. Rev. Neurosci. 22, 219–240 (1999).
Jackson-Lewis, V., Jakowec, M., Burke, R.E. & Przedborski, S. Time course and morphology of dopaminergic neuronal death caused by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neurodegeneration 4, 257– 269 (1995).
Xia, Y. & Zweier, J.L. Superoxide and peroxynitrite generation from inducible nitric oxide synthase in macrophages. Proc. Natl. Acad. Sci. USA 94, 6954–6958 (1997).
Murphy, S. et al. Synthesis of nitric oxide in CNS glial cells. Trends Neurosci. 16, 323–328 (1993).
Galea, E., Feinstein, D.L. & Reis, D.J. Induction of calcium-independent nitric oxide synthase activity in primary rat glial cultures. Proc. Natl. Acad. Sci. USA 89, 10945–10949 (1992).
Garcion, E. et al. Expression of inducible nitric oxide synthase during rat brain inflammation: Regulation by 1,25-dihydroxyvitamin D3. Glia 22, 282–294 (1998).
Dugas, N. et al. Regulation by endogenous interleukin 10 of the expression of nitric oxide synthase induced after ligation of CD23 in human macrophages. Cytokine. 10, 680–689 (1998).
Hunot, S. et al. FcεRII/CD23 is expressed in Parkinson's disease and induces, in vitro, production of nitric oxide and tumor necrosis factor-alpha in glial cells. J. Neurosci. 19, 3440– 3447 (1999).
MacMicking, J., Xie, Q.-W. & Nathan, C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 15, 323–350 (1997).
Halliwell, B. & Gutteridge, J.M. in Free Radicals in Biology and Medicine (Clarendon, Oxford, 1991).
MacMicking, J. et al. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell 81, 641–650 (1995).
Nathan, C. & Root, R.K. Hydrogen peroxide release from mouse peritoneal macrophages. Dependence on sequential activation and triggering. J. Exp. Med. 146, 1648– 1662 (1977).
Leonard, C.S., Kerman, I., Blaha, G., Taveras, E. & Taylor, B. Interdigitation of nitric oxide synthase-, tyrosine hydroxylase-, and serotonin-containing neurons in and around the laterodorsal and pedunculopontine tegmental nuclei of the guinea pig. J. Comp. Neurol. 362, 411–432 (1995).
Dawson, T.M. & Snyder, S.H. Gases as biological messengers: nitric oxide and carbon monoxide in the brain. J. Neurosci. 14, 5147–5159 (1994).
Beckman, J.S. et al. Kinetics of superoxide dismutase- and iron-catalyzed nitration of phenolics by peroxynitrite. Arch. Biochem. Biophys. 298, 438–445 (1992).
Ara, J. et al. Inactivation of tyrosine hydroxylase by nitration following exposure to peroxynitrite and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Proc. Natl. Acad. Sci. USA 95, 7659– 7663 (1998).
Almer, G., Vukosavic, S., Romero, N. & Przedborski, S. Inducible nitric oxide synthase upregulation in a transgenic mouse model of familial amyotrophic lateral sclerosis. J. Neurochem. 72, 2415–2425 (1999).
Asmus, S.E. & Newman, S.W. Colocalization of tyrosine hydroxylase and Fos in the male Syrian hamster brain following different states of arousal. J. Neurobiol. 25, 156– 168 (1994).
Mandir, A.S. et al. Poly (ADP-ribose) polymerase activation mediates MPTP-induced parkinsonism. Proc. Natl. Acad. Sci. USA 96, 5774–5779 (1999).
Coggeshall, R.E. & Lekan, H.A. Methods for determining numbers of cells and synapses: a case for more uniform standards of review. J. Comp. Neurol. 364, 6– 15 (1996).
West, M.J. New stereological methods for counting neurons. Neurobiol. Aging 14, 275–285 (1993).
Ye, Y.Z., Strong, M., Huang, Z.-Q. & Beckman, J.S. in Nitric Oxide. Physiological and Pathological Processes (ed. Packer, L.) 201– 209 (Acedemic, New York, 1996).
Acknowledgements
We thank V.H. Perry and J. Goldman for their advice on the glial studies; H. Ischiropoulos for his guidance on the nitrotyrosine studies; and N. Romero and D. Guestella for their assistance in maintaining and genotyping the iNOS mutant mice. This study was supported by National Institute of Neurological Disorders and Stroke grant R29 NS37345, RO1 NS38586 and P50 NS38370, and US Department of Defense contract number DAMD 17-99-1-9474 (S.P.); the Lowenstein Foundation and the Parkinson's Disease Foundation (G.L., V.J-L., S.P.); National Institutes of Health/National Institute of Neurological Disorders and Stroke grant P50 NS38377; the Mitchell Family foundation (A.S.M, V.L.D, T.M.D); Public Health Service/Career Investigator Development Award grant NS 1K08N2035; and the American Parkinson Disease Association (A.S.M). M.V. is recipient of a fellowship from the Human Frontier Science Program Organization, and S.P. is recipient of the Cotzias Award from the American Parkinson Disease Association.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liberatore, G., Jackson-Lewis, V., Vukosavic, S. et al. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat Med 5, 1403–1409 (1999). https://doi.org/10.1038/70978
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/70978
This article is cited by
-
Beneficial role of capsaicin through modulation of mitochondrial functions in MPTP-injected mice
Neuroscience and Behavioral Physiology (2024)
-
Integrin Mac1 mediates paraquat and maneb-induced learning and memory impairments in mice through NADPH oxidase–NLRP3 inflammasome axis-dependent microglial activation
Journal of Neuroinflammation (2023)
-
Neuroprotective effects of GSK-343 in an in vivo model of MPTP-induced nigrostriatal degeneration
Journal of Neuroinflammation (2023)
-
PSMC5 regulates microglial polarization and activation in LPS-induced cognitive deficits and motor impairments by interacting with TLR4
Journal of Neuroinflammation (2023)
-
18β-glycyrrhetinic acid ameliorates MPTP-induced neurotoxicity in mice through activation of microglial anti-inflammatory phenotype
Psychopharmacology (2023)