Abstract
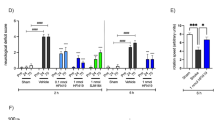
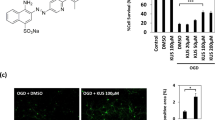
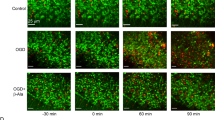
We describe here a new strategy for the treatment of stroke, through the inhibition of NAALADase (N-acetylated-α-linked-acidic dipeptidase), an enzyme responsible for the hydrolysis of the neuropeptide NAAG (N-acetyl-aspartyl-glutamate) to N-acetyl-aspartate and glutamate. We demonstrate that the newly described NAALADase inhibitor 2-PMPA (2-(phosphonomethyl)pentanedioic acid) robustly protects against ischemic injury in a neuronal culture model of stroke and in rats after transient middle cerebral artery occlusion. Consistent with inhibition of NAALADase, we show that 2-PMPA increases NAAG and attenuates the ischemia-induced rise in glutamate. Both effects could contribute to neuroprotection. These data indicate that NAALADase inhibition may have use in neurological disorders in which excessive excitatory amino acid transmission is pathogenic.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Benveniste, H., Drejer, J., Schousboe, A. & Diemer, N.H. Elevation of the extracellular concentrations of glutamate and aspartate in rat hippocampus during transient cerebral ischemia monitored by intracerebral microdialysis. J. Neurochem. 43, 1369–1374 (1984).
Butcher, S.P., Bullock, R., Graham, D.I. & McCulloch, J. Correlation between amino acid release and neuropathologic outcome in rat brain following middle cerebral artery occlusion. Stroke 21, 1727–1733 (1990).
Fagg, G.E. & Foster, A.C. Excitatory amino acid synaptic mechanisms and neurological function. Trends Pharmacol. Sci. 7, 357–363 (1986).
Rothman, S.M. & Olney, J.W. Glutamate and the pathophysiology of hypoxic-ischemic brain death. Ann. Neurol. 19, 105–111 (1986).
Choi, D.W. & Rothman, S.M. The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annu. Rev. Neurosci. 13, 171–182 (1990).
Meldrum, B.S. Protection against ischaemic neuronal damage by drugs acting on excitatory neurotransmission. Cerebrovasc. Brain Metab. Rev. 2, 27–57 (1990).
Wahlgren, N.G. in International Review of Neurobiology: Neuroprotective Agents and Cerebral Ischemia (eds. Green, A.R. & Cross, A.J.) 337–363 (Academic, San Diego, 1997).
Coyle, J.T. et al. in Excitatory Amino Acids (eds. Meldrum, B.S., Moroni, F., Simon, R.P. & Woods, J.H.) 69–77 (Raven, New York, 1991).
Coyle, J.T. The nagging question of the function of N-acetylaspartylglutamate. Neurobiol. Dis. 4, 231–238 (1997).
Tsai, G., Stauch, B.L., Vornov, J.J., Deshpande, J.K. & Coyle, J.T. Selective release of N- acetylaspartylglutamate from rat optic nerve terminals in vivo. Brain Res. 518, 313–316 (1991).
Wrobleska, B. et al. NAAG selectively activates mGluR3 receptors in transfected cells. J. Neurochem. 69, 174–181 (1997).
Vallivullah, H.M., Lancaster, J., Sweetnam, P.M. & Neale, J.H. High concentrations of N- acetylaspartylglutamate and AMPA, kainate, and NMDA binding sites. J. Neurochem. 63, 1714–1719 (1994).
Sekiguchi, M., Okamoto, K. & Sakai, Y. Low concentration N-acetylaspartylglutamate suppresses the climbing fiber response of Purkinje cells in Guinea pig cerebellar slices and the responses to excitatory amino acids of Xenopus laevis oocytes injected with cerebellar mRNA. Brain Res. 482, 87–96 (1989).
Puttfarcken, P.S., Montgomery, D., Coyle, J.T. & Werling, L.L. N-acetyl-L-aspartyl-L-glutamate (NAAG) modulation of NMDA-stimulated [3H]norepinephrine release from rat hippocampal slices. Pharmacol. Exp. Ther. 266, 796–803 (1993).
Slusher, B.S. et al. Rat brain N-acetylated-α-linked acidic dipeptidase activity. Purification and immunologic characterization. J. Biol. Chem. 265, 21297–21301 (1990).
Robinson, M.B., Blakely, R.D., Couto, R. & Coyle, J.T. Hydrolysis of the brain dipeptide N-acetyl- L-aspartyl-L-glutamate. Identification and characterization of a novel N-acetylated alpha-linked acidic dipeptidase activity from rat brain. J. Biol. Chem. 262, 14498–14506 (1987).
Stauch-Slusher, B., Robinson, M.B., Forloni, G., Tsai, G. & Coyle, J.T. The effects of N-acetylated-α-linked acidic dipeptidase (NAALADase) inhibitors on [3H]NAAG catabolism in vivo. Neuro. Sci. Lett. 100, 295–300 (1989).
Fuhrman, S., Palkovits, M., Cassidy, M. & Neale, J.H. The regional distribution of N- acetylaspartylglutamate (NAAG) and peptidase activity against NAAG in the rat nervous system. J. Neurochem. 62, 275–281 (1994).
Meyerhoff, J.L., Carter, R.E., Yourick, D.L., Stauch-Slusher, B. & Coyle, J.T. Genetically epilepsy- prone rats have increased brain regional activity of an enzyme which liberates glutamate from N-acetylaspartylglutamate. Brain Res. 593, 140–143 (1992).
Bruno, V. et al. Activation of class II or III metabotropic glutamate receptors protects cultured cortical neurons against excitotoxic degeneration. Eur. J. Pharm. 7, 1906–1913 (1995).
Serval, V. et al. Competitive inhibition of N-acetylated-α-linked acidic dipeptidase activity by N-acetyl-L-aspartyl-α-linked-L-glutamate. J. Neurochem. 55, 39–46 (1990).
Jackson, P.F. et al. Design, synthesis, and biological activity of a potent inhibitor of the neuropeptidase N-acetylated-α-linked acidic dipeptidase. J. Med. Chem. 39, 619–622 (1996).
Vornov, J.J. Toxic NMDA-receptor activation occurs during recovery in a tissue culture model of ischemia. J. Neurochem. 65, 1681–1691 (1995).
Vornov, J.J., Thomas, A.G. & Jo, D. Protective effects of extracellular acidosis and blockade of sodium/hydrogen ion exchange during recovery from metabolic inhibition in neuronal tissue culture. J. Neurochem. 67, 2379–2389 (1996).
Takahashi, H. et al. PPBP [4- phenyl-1-(4-phenylbutyl)piperidine] decreases brain injury after transient focal ischemic in rats. Stroke 27, 2120–2123 (1996).
Britton, P., Lu, M.X.C., Laskosky, M.S. & Tortella, F.C. Dextromethorphan protects against cerebral injury following transient, but not permanent, focal ischemia in rats. Life Sci. 60 (20), 1729–1740 (1997).
Longa, E.Z., Weinstein, P.R., Carlson, S. & Cummins, R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20: 84–91 (1989).
Small, D.L. & Buchan, A.M. in International Review of Neurobiology: Neuroprotective Agents and Cerebral Ischemia (eds. Green, A.R. & Cross, A.J.) 137–171 (Academic, San Diego, 1997).
Britton, P., Whitton, P.S., Fowler, L.J. & Bowery, N.G. Tetanus toxin-induced effects on extracellular amino acid levels in rat hippocampus: an in vivo microdialysis study. J. Neurochem. 67, 324–329 (1996).
Robinson, M.B., Djali, S. & Buchhalter, J.R. Inhibition of glutamate uptake with L-trans- pyrrolidine-2,4-dicarboxylate potentiates glutamate toxicity in primary hippocampal cultures. J. Neurochem. 61, 2099–2103 (1993).
Zollinger, M., Amsler, U. & Brauchli, J. Quantification of N-acetylaspartylglutamate, an N- terminal blocked dipeptide neurotransmitter candidate, in brain slices superfusates by gas chromatography-mass spectrometry. J. Chromatogr. 532 (1), 27–36 (1990).
Goda, H. et al. Modulation of ischemia-evoked release of excitatory and inhibitory amino acids by adenosine A1 receptor agonists. Eur. J. Pharm. 357, 149–155 (1998).
Muir, K.W. & Lees, K.R. A randomized, double-blind, placebo controlled ascending dose tolerance study of 619C89 in acute stroke. Stroke 26, 503–513 (1995).
Wozniak, D.F. et al. MK-801 neurotoxicity in male mice: histologic effects and chronic impairment in spatial learning. Brain Res. 707, 165–179 (1996).
Fix, A.S., Ross, J.F., Stitzel, S.R. & Switzer, R.C. Integrated evaluation of central nervous system lesions: stains for neurons, astrocytes and microglia reveal the spatial and temporal features of MK-801-induced neuronal necrosis in the rat cerebral cortex. Toxicol. Pathol. 24(3), 291–304 (1996).
Chen, J., Graham, S.H. & Simon, R.P. A comparison of the effects of a sodium channel blocker and an NMDA antagonist upon extracellular glutamate in rat focal ischemia. Brain Res. 699, 121–124 (1995).
Johansen, P.A. et al. Type 4A metabotropic glutamate receptor: identification of new potent agonists and differentiation from the L-(+)-2-amino-4-phosphonobutanoic acid-sensitive receptor in the lateral perforant pathway in rats. Mol. Pharm. 48, 140–149 (1995).
Thomsen, C., Boel, E. & Suzdak, P.D. Actions of phenylglycine analogs at subtypes of the metabotropic glutamate receptor family. Eur. J. Pharm. 267, 77–84 (1994).
Acknowledgements
The authors thank S. Snyder for his insight and discussions in the preparation of this manuscript and E. Lea and E. Lisska for their assistance in the amino-acid analyses. All experiments were supported by Guilford Pharmaceuticals.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Slusher, B., Vornov, J., Thomas, A. et al. Selective inhibition of NAALADase, which converts NAAG to glutamate, reduces ischemic brain injury. Nat Med 5, 1396–1402 (1999). https://doi.org/10.1038/70971
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/70971
This article is cited by
-
Characterization of glutamate carboxypeptidase 2 orthologs in trematodes
Parasites & Vectors (2022)
-
High uptake of 68Ga-PSMA and 18F-DCFPyL in the peritumoral area of rat gliomas due to activated astrocytes
EJNMMI Research (2020)
-
Looking for Drugs in All the Wrong Places: Use of GCPII Inhibitors Outside the Brain
Neurochemical Research (2020)
-
NAAG Peptidase Inhibitors Act via mGluR3: Animal Models of Memory, Alzheimer’s, and Ethanol Intoxication
Neurochemical Research (2017)
-
N-acetylaspartylglutamate Inhibits Heroin Self-Administration and Heroin-Seeking Behaviors Induced by Cue or Priming in Rats
Neuroscience Bulletin (2017)