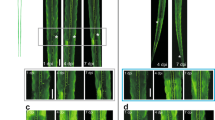
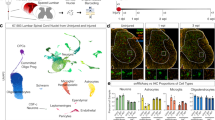
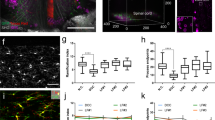
Abstract
The poor response of central axons to transection underlies the bleak prognosis following spinal cord injury. Here, we monitor individual fluorescent axons in the spinal cords of living transgenic mice over several days after spinal injury. We find that within 30 min after trauma, axons die back hundreds of micrometers. This acute form of axonal degeneration is similar in mechanism to the more delayed Wallerian degeneration of the disconnected distal axon, but acute degeneration affects the proximal and distal axon ends equally. In vivo imaging further shows that many axons attempt regeneration within 6–24 h after lesion. This growth response, although robust, seems to fail as a result of the inability of axons to navigate in the proper direction. These results suggest that time-lapse imaging of spinal cord injury may provide a powerful analytical tool for assessing the pathogenesis of spinal cord injury and for evaluating therapies that enhance regeneration.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ramón y Cajal, S. Degeneration and regeneration of white matter in Cajal's Degeration and Regeneration of the Nervous System (eds DeFelipe, J. & Jones, E.G.) ch.4 (Oxford Univ. Press, New York, 1991).
Steward, O., Zheng, B. & Tessier-Lavigne, M. False resurrections: distinguishing regenerated from spared axons in the injured central nervous system. J. Comp. Neurol. 459, 1–8 (2003).
Feng, G. et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 28, 41–51 (2000).
Neumann, S. & Woolf, C.J. Regeneration of dorsal column fibers into and beyond the lesion site following adult spinal cord injury. Neuron 23, 83–91 (1999).
Qiu, J. et al. Spinal axon regeneration induced by elevation of cyclic AMP. Neuron 34, 895–903 (2002).
Walsh, M.K. & Lichtman, J.W. In vivo time-lapse imaging of synaptic takeover associated with naturally occurring synapse elimination. Neuron 37, 67–73 (2003).
Tom, V.J., Steinmetz, M.P., Miller, J.H., Doller C.M. & Silver J. Studies on the development and behavior of the dystrophic growth cone, the hallmark of regeneration failure, in an in vitro model of the glial scar and after spinal cord injury. J. Neurosci. 24, 6531–6539 (2004)
Firkins, S.S., Bates, C.A., & Stelzner, D.J. Corticospinal tract plasticity and astroglial reactivity after cervical spinal injury in the postnatal rat. Exp. Neurol. 120, 1–15 (1993).
Demjen, D. et al. Neutralization of CD95 ligand promotes regeneration and functional recovery after spinal cord injury. Nat. Med. 10, 389–395 (2004).
Houle, J.D. & Jin, Y. Chronically injured supraspinal neurons exhibit only modest axonal dieback in response to a cervical hemisection lesion. Exp. Neurol. 169, 208–217 (2001).
Perry, V.H., Brown, M.C. & Lunn, E.R. Very slow retrograde and Wallerian degeneration in the CNS of C57BL/Ola mice. Eur. J. Neurosci. 3, 102–105 (1991).
Mack, T.G. et al. Wallerian degeneration of injured axons and synapses is delayed by a Ube4b/Nmnat chimeric gene. Nat. Neurosci. 4, 1199–1206 (2001).
George, E.B., Glass, J.D. & Griffin, J.W. Axotomy-induced axonal degeneration is mediated by calcium influx through ion-specific channels. J. Neurosci. 15, 6445–6452 (1995).
Zhai, Q. et al. Involvement of the ubiquitin-proteasome system in the early stages of Wallerian degeneration. Neuron 39, 217–225 (2003).
Saido, T.C. et al. Spatial resolution of fodrin proteolysis in postischemic brain. J. Biol. Chem. 268, 25239–25243 (1993).
Hill, C.E., Beattie, M.S. & Bresnahan, J.C. Degeneration and sprouting of identified descending supraspinal axons after contusive spinal cord injury in the rat. Exp. Neurol. 171, 153–169 (2001).
Li, Y. & Raisman, G. Sprouts from cut corticospinal axons persist in the presence of astrocytic scarring in long-term lesions of the adult rat spinal cord. Exp. Neurol. 134, 102–111 (1995).
Pan, Y.A., Misgeld, T., Lichtman J.W. & Sanes, J.R. Effects of neurotoxic and neuroprotective agents on peripheral nerve regeneration assayed by time-lapse imaging in vivo. J. Neurosci. 23, 11479–11488 (2003)
Raff, M.C., Whitmore, A.V. & Finn, J.T. Axonal self-destruction and neurodegeneration. Science 296, 868–871 (2002).
Bareyre, F.M. et al. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat. Neurosci. 7, 269–277 (2004).
Nguyen, Q.T., Sanes, J.R. & Lichtman, J.W. Pre-existing pathways promote precise projection patterns. Nat. Neurosci. 5, 861–867 (2002).
Beirowski, B. et al. Quantitative and qualitative analysis of Wallerian degeneration using restricted axonal labelling in YFP-H mice. J. Neurosci. Meth. 134, 23–35 (2004).
Gillingwater, T.H. et al. Age-dependent synapse withdrawal at axotomised neuromuscular junctions in Wld(s) mutant and Ube4b/Nmnat transgenic mice. J. Physiol. 543, 739–755 (2002).
Acknowledgements
The authors wish to thank J. Sanes for providing GFP-S mice and support, T.C. Saido for providing the antibody specific for spectrin degradation products, J. Tollett for animal husbandry, J. Lu for writing software, L. Godinho, F. Bareyre and J. Sanes for suggestions and critical reading of the manuscript. This work was supported by grants from the Christopher Reeve Paralysis Foundation (T.M.), the Emmy Noether Program of the Deutsche Forschungsgemeinschaft (T.M., M.K.) and grants from the Swiss National Science Foundation (M.E.S.) and the US National Institutes of Health (J.W.L.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Wallerian degeneration. (PDF 81 kb)
Supplementary Fig. 2
Acute axonal degeneration is reduced in WLD-S mice. (PDF 90 kb)
Supplementary Fig. 3
Calpain mediates acute axonal degeneration. (PDF 286 kb)
Supplementary Fig. 4
Neurofilament staining in acute axonal degeneration. (PDF 379 kb)
Supplementary Fig. 5
Nodal sprout during early axonal out-growth. (PDF 88 kb)
Supplementary Fig. 6
Reconstruction of a sprouting DRG neuron. (PDF 120 kb)
Rights and permissions
About this article
Cite this article
Kerschensteiner, M., Schwab, M., Lichtman, J. et al. In vivo imaging of axonal degeneration and regeneration in the injured spinal cord. Nat Med 11, 572–577 (2005). https://doi.org/10.1038/nm1229
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1229
This article is cited by
-
Electroacupuncture Inhibits Neuroinflammation Induced by Astrocytic Necroptosis Through RIP1/MLKL/TLR4 Pathway in a Mouse Model of Spinal Cord Injury
Molecular Neurobiology (2023)
-
Methylene blue for intractable pain from oral mucositis related to cancer treatment: a randomized phase 2 clinical trial
BMC Medicine (2022)
-
Long-term in vivo imaging of mouse spinal cord through an optically cleared intervertebral window
Nature Communications (2022)
-
Mitochondrial function in spinal cord injury and regeneration
Cellular and Molecular Life Sciences (2022)
-
The Histopathology of Severe Graded Compression in Lower Thoracic Spinal Cord Segment of Rat, Evaluated at Late Post-injury Phase
Cellular and Molecular Neurobiology (2022)