Abstract
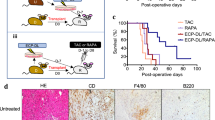
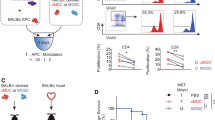
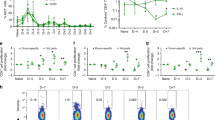
With an organ transplant, hematopoietic donor cells are transferred to the recipient. To study the relevance of the resulting microchimerism for allograft acceptance, we analyzed a rat model of cyclosporine-induced tolerance for strongly incompatible heart allografts. Using a monoclonal antibody that detects a donor-specific CD45 allotype (RT7a), we selectively depleted donor leukocytes at different times after transplantation (days 0 or 18). Depletion was similarly effective at both times. However, only depletion on day 0 prevented tolerance induction and was associated with severe acute or chronic graft rejection. This indicates that passenger leukocytes have an essential immunomodulatory effect on the induction phase of allograft acceptance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Starzl, T.E. et al. Cell migration, chimerism, and graft acceptance. Lancet 339, 1579–1582 (1992).
Starzl T.E. et al. Cell migration and chimerism after whole organ transplantation: The basis of graft acceptance. Hepatology 17, 1127–1152 (1993).
Starzl, T.E. et al. Donor cell chimerism permitted by immunosuppressive drugs: a new view of organ transplantation. Immunol. Today 14, 326–332 (1993).
Starzl, T.E. & Zinkernagel, R.M. Antigen localization and migration in immunity and tolerance. N. Engl. J. Med. 339, 1905–1913 (1998).
McSherry, C. et al. Sequential measurement of peripheral blood allogeneic microchimerism levels and association with pulmonary function. Transplantation 62, 1811–1818 (1996).
Wood, K. & Sachs, D.H. Chimerism and transplantation tolerance: cause and effect. Immunol. Today 17, 584–587 (1996).
Schlitt, H.J. et al. Patterns of donor-type microchimerism after heart transplantation. Lancet 343, 1469–1471 (1994).
Schlitt, H.J., Hundrieser, J., Ringe, B. & Pichlmayr, R. Systemic microchimerism of donor-type associated with irreversible acute liver graft rejection eight years after transplantation. N. Engl. J. Med. 330, 646–647 (1994).
Hisanaga, M. et al. Development, stability, and clinical correlations of allogeneic microchimerism after solid organ transplantation. Transplantation 61, 40–45 (1996).
Wonigeit, K. Characterization of the RT-Ly-1 and RT-Ly-2 alloantigenic systems by congenic rat strains. Transplant. Proc. 11, 1631–1635 (1979).
Kampinga, J. et al. RT7-defined alloantigens in rats are part of the leukocyte common antigen family. Scand. J. Immunol. 31, 699–710 (1990).
Heidecke, C.-D. et al. α/β-T cell receptor-directed therapy in rat allograft recipients. Transplantation 61, 948–956 (1996).
Lechler, R.I. & Batchelor, J.R. Restoration of immunogenicity to passenger cell-depleted kidney allografts by the addition of donor strain dendritic cells. J. Exp. Med. 155, 31–41 (1982).
Sun, J. et al. Deletion of spontaneous rat liver allograft acceptance by donor irradiation. Transplantation 60, 233–236 (1995).
Schlitt, H.J. Is microchimerism needed for allograft tolerance? Transplant. Proc. 29, 82–84 (1997).
Ueda, M. et al. Development of microchimerism in pediatric patients after living-related liver transplantation. Clin. Transplant. 11, 193–199 (1997).
Schlitt, H.J. et al. Persistence of donor lymphocytes in liver allograft recipients. Transplantation 56, 1001–1007 (1993).
Larsen, C.P, Morris, P. & Ausytn, J.M. Migration of dendritic leukocytes from cardiac allografts into host spleens: a novel pathway for initiation of rejection. J. Exp. Med. 171, 307–314 (1990).
Murase, N. et al. Effect in supralethally irradiated rats of granulocyte colony-stimulating factor and lisofylline on hematopoietic reconstitution by syngeneic bone marrow or whole organ passenger leukocytes. Transplantation 63, 1840–1843 (1997).
Demetris, A.J. et al. Hematolymphoid cell trafficking, microchimerism, and GVH reactions after liver, bone marrow, and heart transplantation. Transplant. Proc. 25, 3337–3344 (1993).
Santin, A.D. et al. The effects of irradiation on the expression of a tumour rejection antigen (heat shock protein gp96) in a human cervical cancer. Int. J. Radiat. Biol. 73, 699–704 (1998).
Sriwatanawongsa, V., Davies, H.ff., & Calne, R.Y. The essential roles of parenchymal tissues and passenger leukocytes in the tolerance induced by liver grafting in rats. Nature Med. 1, 428–432 (1995).
Ehl, S. et al. Antigen persistence and time of T-cell tolerization determine the efficacy of tolerization protocols for prevention of skin graft rejection. Nature Med. 4, 1015–1019 (1998).
Schwarz, R.H. A cell culture model for T lymphocyte clonal anergy. Science 248, 1349–1356 (1990).
Lombardi, G. et al. Antigen presentation by T cells inhibits IL-2 production and induces IL-4 release due to altered cognate signals. J. Immunol. 156, 2769–2775 (1996).
Tsui, T.Y., Deiwick, A., Ko, S. & Schlitt, H.J. Specific immunosuppression by postoperative infusion of allogeneic spleen cells. Requirement of donor MHC expression and graft-versus-host reactivity. Transplantation (in the press).
Thomas, J.M. et al. Veto cells in transplantation tolerance. Clin. Transplant. 8, 195–203 (1994).
Thomas, J.M. Further studies of veto activity in rhesus monkey bone marrow in relation to allograft tolerance and chimerism. Transplantation 57, 101–115 (1994).
Raddatz, G., Deiwick, A., Sato, T. & Schlitt, H.J. Inhibition of cytotoxic alloreactivity by human allogeneic mononuclear cells: evidence for veto function of CD2+ cells. Immunology 94, 101–108 (1998).
Tomita, Y., Khan, A. & Sykes, M. Role of intrathymic clonal deletion and peripheral anergy in transplantation tolerance induced by bone marrow transplantation in mice conditioned with a nonmyeloablative regimen. J. Immunol. 153, 1087–1098 (1994).
Khan, A., Tomita, Y. & Sykes, M. Thymic dependence of loss of tolerance in mixed allogeneic chimeras after depletion of donor antigen. Transplantation 62, 380–387 (1996).
Ono, K. & Lindsey, E.S. Improved technique of heart transplantation in rats. J. Thorac. Cardiovasc. Surg. 57, 225–229 (1969).
Schwinzer, R., Hedrich H.-J. & Wonigeit, K. T cell differentiation in athymic nude rats (rnu/rnu): demonstration of a distorted T cell subset structure by flow cytometry analysis. Eur. J. Immunol. 19, 1841–1847 (1989).
Hoffman, R.A. et al. Alloantigen-induced activation of rat splenocytes is regulated by the oxidative metabolism of L-arginine. J. Immunol. 145, 2220–2226 (1990).
Wood, P.J. et al. Prevention of chronic rejection by donor-specific blood transfusion in a new model of chronic cardiac allograft rejection. Transplantation 61, 1440–1443 (1996).
Demetris, A.J. et al. Analysis of chronic rejection and obliterative arteriopathy. Am. J. Pathol. 150, 563–578 (1997).
Schmid, C., Heemann, U. & Tilney, N.L. Retransplantation reverses mononuclear infiltration but not myointimal proliferation in a rat model of chronic cardiac allograft rejection. Transplantation 61, 1695–1699 (1996).
Strehlau, J. et al. The intragraft gene activation of markers reflecting T-cell-activation and -cytotoxicity analysed by quantitative RT-PCR in renal transplantation. Clin. Nephrol. 46, 30–33 (1996).
Siebert, P.D. & Larrick, J.W. PCR MIMICS: competitive DNA fragments for use as internal standards in quantitative PCR. BioTechniques 14, 244–249 (1993).
Siegling, A. et al. A novel multispecific competitor fragment for quantitative PCR analysis of cytokine gene expression in rats. J. Immunol. Method 177, 23–28 (1994).
Acknowledgements
The authors thank L. Hänisch for animal care, M. Giesecke for microsurgical assistance and M.W. Hoffmann for critical review of the manuscript. This work was supported by the Deutsche Forschungsgemeinschaft through Sonderforschungsbereich 265. S.K. was supported by a fellowship of the Alexander von Humboldt Stiftung; his present affiliation is First Department of Surgery, Nara Medical University, Kashihara, Nara 634, Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ko, S., Deiwick, A., Jäger, M. et al. The functional relevance of passenger leukocytes and microchimerism for heart allograft acceptance in the rat. Nat Med 5, 1292–1297 (1999). https://doi.org/10.1038/15248
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/15248
This article is cited by
-
Targeting the niche: depleting haemopoietic stem cells with targeted therapy
Bone Marrow Transplantation (2019)
-
Transplantation tolerance through mixed chimerism
Nature Reviews Nephrology (2010)
-
Adenoviral transfer of a single donor-specific MHC class I gene to recipient bone marrow cells can induce specific immunological unresponsiveness in vivo
Gene Therapy (2002)
-
Immunity or tolerance: Opposite outcomes of microchimerism from skin grafts
Nature Medicine (2001)
-
Superior survival of blood and marrow stem cell recipients given maternal grafts over recipients given paternal grafts
Bone Marrow Transplantation (2001)