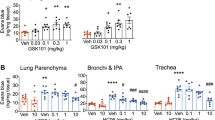
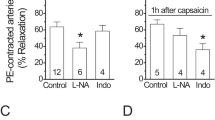
Abstract
Plasma extravasation from postcapillary venules is one of the earliest steps of inflammation1. Substance P (SP) and bradykinin (BK) mediate extravasation and cause hypotension2–6. The cell-surface enzyme neutral endopeptidase (NEP) inactivates both peptides7,8. Thus, absence of NEP may predispose development of inflammation and hypotension. We examined these possibilities in mice in which the NEP gene was deleted by homologous recombination9. There was widespread basal plasma extravasation in postcapillary venular endothelia in NEP−/− mice, which was reversed by recombinant NEP and antagonists of SP (NK1) and BK (B2) receptors. Mean arterial blood pressure was 20% lower in NEP−/− animals, but this was unaffected by reintroduction of recombinant NEP and the kinin receptor antagonists. The hypotension was also independent of nitric oxide (NO), because NEP−/− mice treated with a NO synthase inhibitor remained hypotensive relative to the wild type. Thus, NEP has important roles in regulating basal microvascular permeability by degrading SP and BK, and may regulate blood pressure set point through a mechanism that is independent of SP, BK and NO. The use of NEP antagonists as candidate drugs in cardiovascular disease is suggested by the blood pressure data reported herein.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Payan, D., Substance, P. in Inflammation: Basic Principles and Clinical Correlates (eds. Gallin, J.I., Goldstein, I.M. & Snyderman, R.) 177–191 (Raven, New York, 1992).
Von Euler, U.S. & Gaddum, J.H. An unidentified depressor substance in certain tissue extracts. J. Physiol. (Lond.) 72, 74–87 (1931).
Lembeck, F. & Holzer, P. Substance P as neurogenic mediator of antidromic vasodilation and neurogenic plasma extravasation. Naunyn-Schmiedeberg's Arch. Pharmacol. 310, 175–183 (1979).
Lembeck, F. et al. The non-peptide tachykinin antagonist, CP-96, 345, is a potent inhibitor of neurogenic inflammation. Br. J. Pharmacol. 105, 527–30 (1992).
Otsuka, M. & Yoshioka, K. Neurotransmitter functions of mammalian tachykinins. Physiol. Rev. 73, 229–308 (1993).
Bhoola, K.D., Figueroa, C.D. & Woerthy, K. Bioregulation of kinins: Kallikreins, kininogens, and kininases. Pharmacol. Rev. 44, 1–80 (1992).
Matsas, R. et al. Substance P and [Leu]enkephalin are hydrolyzed by an enzyme in pig caudate synaptic membranes that is identical with the endopeptidase of kidney microvilli. Proc. Natl. Acad. Sci. USA 80, 3111–3115 (1983).
Matsas, R., Kenny, A.J. & Turner, A.J. The metabolism of neuropeptides: The hydrolysis of peptides, including enkephalins, tachykinins and their analogues, by endopeptidase-24.11. Biochem. J. 233, 433–440 (1984).
Lu, B. et al. Neutral endopeptidase modulation of septic shock. J. Exp. Med. 181, 2271–2275 (1995).
Geppetti, P. Sensory neuropeptide release by bradykinin: Mechanisms and patho-physiological implications. Regal. Pept. 47, 1–23 (1993).
Gee, N.S. et al. An immunoradiometric assay for endopeptidase-24.11 shows it to be a widely distributed enzyme in pig tissues. Biochem. J. 228, 119–126 (1985).
Matsas, R., Kenny, A.J. & Turner, A.J. An immunohistochemical study of endopeptidase-24.11 (‘enkephalinase’) in the pig nervous system. Neuroscience 18, 991–1012 (1986).
Bunnett, N.W. et al. Distribution and abundance of neutral endopeptidase (EC 3.4.24.11) in the alimentary tract of the rat. Am. J. Physiol. 264, G497G508 (1993).
Umeno, E. et al. Inhibition of the neutral endopeptidase potentiates neurogenic inflammation in the rat trachea. J. Appl. Physiol. 66, 2647–2652 (1989).
Lotvall, J.O. et al. Bradykinin-induced airway microvascular leakage is potentiated by captopril and phosphoramidon. Eur. J. Pharmacol. 200, 211–7 (1991).
Saria, A. & Lundberg, J.M. Evans blue fluorescence: Quantitative and morphological evaluation of vascular permeability in animal tissues. J. Neurosci. Methods 8, 41–49 (1983).
Joris, I. et al. Vascular labelling with Monastral blue B. Stain Technol. 57, 177–183 (1982).
Emonds-Alt, X. et al. In vitro and in vivo biological activities of SR140333, a novel potent non-peptide tachykinin NK1 receptor antagonist. Eur. J. Pharmacol. 250, 403–413 (1993).
Rhaleb, N.E. et al. Pharmacological characterization of a new highly potent B2 receptor antagonist (HOE 140: D-Arg-[Hyp3, Thi5, D-Tic7, Qic8] bradykinin). Eur. J. Pharmacol. 210, 115–120 (1992).
Bunnett, N.W. et al. Metabolism of gastrin and cholecystokinin by endopeptidase 24.11 from the pig stomach. Am. J. Physiol. 255, G676–G684 (1988).
Kenny, A.J. & Stephenson, S.L. Role of endopeptidase-24.11 in the inactivation of atrial natriuretic peptide. FASEB Lett. 232, 1–8 (1988).
Gardiner, S.M. et al. Effects of the neutral endopeptidase inhibitor, SQ 28, 603, on regional haemodynamic responses to atrial natriuretic peptide or proendothelin-1 [1-38] in conscious rats. Br. J. Pharmacol. 106, 180–6 (1992).
Lifton, R.P. Molecular genetics of human blood pressure variation. Science 272, 666–680 (1996).
Hwang, L. et al. Downregulation of neutral endopeptidase (EC 3.4.24.11) in the inflamed rat intestine. Am. J. Physiol. 264, G735–G743 (1993).
Borson, D.B. et al. Neutral endopeptidase and neurogenic inflammation in rats with respiratory infections. J. Appl. Physiol. 66, 2653–2658 (1989).
Figini, M. et al. Substance P and bradykinin stimulate plasma extravasation in the mouse gastrointestinal tract and pancreas. Am. J. Physiol. 272, G785–G793 (1997).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lu, B., Figini, M., Emanueli, C. et al. The control of microvascular permeability and blood pressure by neutral endopeptidase. Nat Med 3, 904–907 (1997). https://doi.org/10.1038/nm0897-904
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nm0897-904
This article is cited by
-
Membranous nephropathy
Nature Reviews Disease Primers (2021)
-
Inflammation: the link between comorbidities, genetics, and Alzheimer’s disease
Journal of Neuroinflammation (2018)
-
Role of neurogenic inflammation in local communication in the visceral mucosa
Seminars in Immunopathology (2018)
-
Long-term neprilysin inhibition — implications for ARNIs
Nature Reviews Cardiology (2017)
-
A podocyte view of membranous nephropathy: from Heymann nephritis to the childhood human disease
Pflügers Archiv - European Journal of Physiology (2017)