Abstract
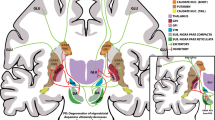
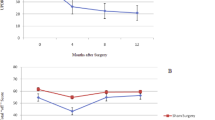
Huntington's disease is an autosomal dominant, inherited disorder that results in progressive degeneration of the basal ganglia (especially the neostriatal caudate nucleus and putamen) and other forebrain structures and is associated with a clinical profile of movement, cognitive and psychiatric impairments for which there is at present no effective therapy1. Neuropathological, neurochemical and behavioral features of the disease can all be reproduced in experimental animals by local injection of excito-toxic2,3 or metabolic4,5 toxins into the neostriatum. All these features of the disease can be alleviated, at least in rats, by transplantation of embryonic striatal tissue into the degenerated striatum6–8, which was the basis for commencing the first clinical trials of striatal transplantation in Huntington's patients9,10. However, although rat striatal xenografts may temporarily reduce apomorphine-induced dyskinesias in monkeys11, there has been no demonstration that allograft techniques that work well in rats translate effectively to the much larger differentiated striatum of primates12. Here we demonstrate good survival, differentiation and integration of striatal allografts in the primate neostriatum, and recovery in a test of skilled motor performance. Long-term graft survival in primates indicates probable success for clinical transplants in Huntington's disease; in addition, our data suggest that graft placement has a direct influence on the pattern and extent of functional recovery.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Harper, P.S. In Huntington's Disease (W.B. Saunders, London, 1996).
Coyle, J.T. & Schwarcz, R. Lesions of striatal neurones with kainic acid provides a model for Huntington's chorea. Nature 263, 244–246 (1976).
Ferrante, R.J., Kowall, N.W., Cipolloni, P.B., Storey, E. & Beal, M.F. Excitotoxin lesions in primates as a model for Huntington's disease: Histopathologic and neuro-chemical characterization. Exp. Neurol. 119, 46–71 (1993).
Beal, M.F. et al. Neurochemical and histologic characterization of striatal excito-toxic lesions produced by the mitochondrial toxin 3-nitropropionic acid. J. Neurosci. 13, 4181–4192 (1993).
Palfi, S.P. et al. Chronic 3-nitropropionic acid treatment in baboons replicates the cognitive and motor deficits of Huntington's disease. J. Neurosci. 16, 3019–3025 (1996).
Björklund, A., Campbell, K., Sirinathsinghji, D.J.S., Fricker, R.A. & Dunnett, S.B., In Functional Neural Transplantation, (eds Dunnett, S.B. & Björklund, A.) 157–195 (Raven Press, New York, 1994)
Dunnett, S.B., Isacson, O., Sirinathsinghji, D.J.S., Clarke, D.J. & Björklund, A. Striatal grafts in rats with unilateral neostriatal lesions. III. Recovery from dopamine-dependent motor asymmetry and deficits in skilled paw reaching. Neuroscience 24, 813–820 (1988).
Wictorin, K. Anatomy and connectivity of intrastriatal striatal transplants. Prog. Neurobiol. 38, 611–639 (1992).
Philpott, L.M. et al. Neuropsychological functioning following fetal striatal transplantation in Huntington's chorea: Three case presentations. Cell Transplant. 6, 203–212 (1997).
Madrazo, I., Franco-Bourland, R.E., Castrejon, H., Cuevas, C. & Ostrosky-Solis, F. Fetal striatal homotransplantation for Huntington's disease: First two case reports. Neurol. Res. 17, 312–315 (1995).
Hantraye, P., Riche, D., Mazière, M. & Isacson, O. Intrastriatal transplantation of cross-species fetal striatal cells reduces abnormal movements in a primate model of Huntington's disease. Proc. Natl. Acad. Sci. USA 89, 4187–4191 (1992).
Helm, G.A., Palmer, P.E., Simmons, N.E., DiPierro, C.C. & Bennett, J.P. Degeneration of long-term fetal neostriatal allografts in the Rhesus monkey: An electron microscopic study. Exp. Neurol. 123, 174–180 (1993).
Marshall, J.W.B. & Ridley, R.M. Assessment of functional impairment following permanent middle cerebral artery occlusion in a nonhuman primate species. Neurodegeneration 5, 275–286 (1996).
Annett, L.E. et al. Behavioural assessment of the effects of embryonic nigral grafts in marmosets with unilateral 6-OHDA lesions of the nigrostriatal pathway. Exp. Neurol. 125, 228–246 (1994).
Graybiel, A.M. et al. Intrastriatal grafts derived from fetal striatal primordia. I. Phenotypy and modular organization. J. Neurosci. 9, 3250–3271 (1989).
Ouimet, C.C., Miller, P.E., Hemmings, H.C., Walaas, S.I. & Creengard, P. DARPP-32, a dopamine- and adenosine-3′,5′-monophosphate-regulated phosphoprotein enriched in dopamine-innervated brain regions. J. Neurosci. 4, 111–124 (1984).
Helm, G.A., Palmer, P.E., Simmons, N.E., DiPierro, C. & Bennett, J.P. Descriptive morphology of developing fetal neostriatal allografts in the rhesus monkey: A correlated light and electron microscopic golgi study. Neuroscience 50, 163–179 (1992).
Isacson, O., Riche, D., Hantraye, P., Sofroniew, M.V. & Mazière, M. A primate model of Huntington's disease: Cross-species implantation of striatal precursor cells to the excitotoxically lesioned baboon caudate-putamen. Exp. Brain Res. 75, 213–220 (1989).
Stephan, H., Baron, R. & Schwerdtfeger, W.K. In The Brain of the Common Marmoset: a Stereotaxic Atlas. (Springer-Verlag, Berlin, 1980).
Dunnett, S.B. & Björklund, A. Neural Transplantation: A Practical Approach. (IRL Press, Oxford, 1992).
Pakzaban, P., Deacon, T.W., Burns, L.H. & Isacson, O. Increased proportion of acetylcholinesterase-rich zones and improved morphological integration in host striatum of fetal grafts derived from the lateral but not the medial ganglionic eminence. Exp. Brain Res. 97, 13–22 (1993).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kendall, A., Rayment, F., Torres, E. et al. Functional integration of striatal allografts in a primate model of Huntington's disease. Nat Med 4, 727–729 (1998). https://doi.org/10.1038/nm0698-727
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nm0698-727
This article is cited by
-
Choosing an animal model for the study of Huntington's disease
Nature Reviews Neuroscience (2013)
-
Selection of suitable reference genes for mRNA quantification studies using common marmoset tissues
Molecular Biology Reports (2013)
-
Quantitative molecular assessment of chimerism across tissues in marmosets and tamarins
BMC Genomics (2012)
-
Human Pluripotent Stem Cell Therapy for Huntington's Disease: Technical, Immunological, and Safety Challenges
Neurotherapeutics (2011)
-
Setback for Huntington's disease therapy
Nature (2009)