Abstract
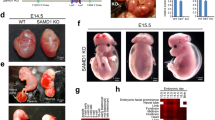
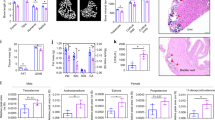
The only proven requirement for ascorbic acid (vitamin C) is in preventing scurvy1,2, presumably because it is a cofactor for hydroxylases required for post-translational modifications that stabilize collagen3. We have created mice deficient in the mouse ortholog (solute carrier family 23 member 1 or Slc23a1) of a rat ascorbic-acid transporter, Svct2 (ref. 4). Cultured embryonic fibroblasts from homozygous Slc23a1−/− mice had less than 5% of normal ascorbic-acid uptake. Ascorbic-acid levels were undetectable or markedly reduced in the blood and tissues of Slc23a1−/− mice. Prenatal supplementation of pregnant females did not elevate blood ascorbic acid in Slc23a1−/− fetuses, suggesting Slc23a1 is important in placental ascorbic-acid transport. Slc23a1−/− mice died within a few minutes of birth with respiratory failure and intraparenchymal brain hemorrhage. Lungs showed no postnatal expansion but had normal surfactant protein B levels. Brain hemorrhage was unlikely to be simply a form of scurvy since Slc23a1−/− mice showed no hemorrhage in any other tissues and their skin had normal skin 4-hydroxyproline levels despite low ascorbic-acid content. We conclude that Slc23a1 is required for transport of ascorbic acid into many tissues and across the placenta. Deficiency of the transporter is lethal in newborn mice, thereby revealing a previously unrecognized requirement for ascorbic acid in the perinatal period.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hodges, R.E., Hood, J., Canham, J.E., Sauberlich, H.E. & Baker, E.M. Clinical manifestations of ascorbic acid deficiency in man. Am J. Clin. Nutr. 24, 432–443 (1971).
Levine, M., Rumsey, S.C., Daruwala, R., Park, J.B. & Wang, Y. Criteria and recommendations for vitamin C intake. JAMA 281. 281, 1415–1423 (1999).
Byers, P.H. Disorders of collagen biosynthesis and structure. in The Metabolic and Molecular Bases of Inherited Disease, vol. IV (eds. Scriver, C.R., Beaudet, A.L., Sly, W.S. &Valle, D.) 5241–5285 (McGraw-Hill, New York, 2001).
Tsukaguchi, H. et al. A family of mammalian Na+-dependent l-ascorbic acid transporters. Nature 399. 399, 70–75 (1999).
Gispert, S., Dutra, A., Lieberman, A., Friedlich, D. & Nussbaum, R.L. Cloning and genomic organization of the mouse gene slc23a1 encoding a vitamin C transporter. DNA Res. 7, 339–345 (2000).
Volpe, J.J. Intraventricular hemorrhage and brain injury in the premature infant. Neuropathology and pathogenesis. Clin. Perinatol. 16, 361–386 (1989).
Goldenberg, H. & Schweinzer, E. Transport of vitamin C in animal and human cells. J. Bioenerg. Biomembr. 26, 359–367 (1994).
Rumsey, S.C. & Levine, M. Absorption, transport, and distribution of ascorbic acid in humans. J. Nutr. Biochem. 9, 116–130 (1998).
Choi, J.L. & Rose, R.C. Transport and metabolism of ascorbic acid in human placenta. Am J. Physiol. 257, C110–113 (1989).
Agus, D.B. et al. Vitamin C crosses the blood-brain barrier in the oxidized form through the glucose transporters. J. Clin. Invest. 100, 2842–2848 (1997).
Daruwala, R., Song, J., Koh, W.S., Rumsey, S.C. & Levine, M. Cloning and functional characterization of the human sodium-dependent vitamin C transporters hSVCT1 and hSVCT2. FEBS Lett. 460, 480–484 (1999).
Rajan, D.P. et al. Human placental sodium-dependent vitamin C transporter (SVCT2): Molecular cloning and transport function. Biochem. Biophys. Res. Commun. 262, 762–768 (1999).
Kratzing, C.C. & Kelly, J.D. Ascorbic acid synthesis by the mammalian fetus. Int. J. Vitam. Nutr. Res. 56, 101–103 (1986).
Rosso, P. & Norkus, E. Prenatal aspects of ascorbic acid metabolism in the albino rat. J. Nutr. 106, 767–70 (1976).
Jenness, R., Birney, E.C., Ayaz, K.L. & Buzzell, D.M. Ontogenetic development of L-gulonolactone oxidase activity in several vertebrates. Comp. Biochem. Physiol. B 78, 167–173 (1984).
Norkus, E.P., Bassi, J. & Rosso, P. Maternal-fetal transfer of ascorbic acid in the guinea pig. J. Nutr. 109, 2205–2212 (1979).
Rumsey, S.C. et al. Glucose transporter isoforms GLUT1 and GLUT3 transport dehydroascorbic acid. J. Biol. Chem. 272, 18982–18989 (1997).
Bass, W.T., Malati, N., Castle, M.C. & White, L.E. Evidence for the safety of ascorbic acid administration to the premature infant. Am. J. Perinatol. 15, 133–140 (1998).
Silvers, K.M., Gibson, A.T. & Powers, H.J. High plasma vitamin C concentrations at birth associated with low antioxidant status and poor outcome in premature infants. Arch. Dis. Child Fetal Neonatal Ed. 71, F4044 (1994).
Hogan, B., Beddington, R., Costantini, F. & Lacy, E. Manipulating the Mouse Embryo: A Laboratory Manual (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, 1994).
Miller, S.A., Dykes, D.D. & Polesky, H.F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 16, 1215 (1988).
Welch, R.W., Bergsten, P., Butler, J.D. & Levine, M. Ascorbic acid accumulation and transport in human fibroblasts. Biochem. J. 294, 505–510 (1993).
Washko, P.W., Wang, Y. & Levine, M. Ascorbic acid recycling in human neutrophils. J. Biol. Chem. 268, 15531–15535 (1993).
Dhariwal, K.R., Hartzell, W.O. & Levine, M. Ascorbic acid and dehydroascorbic acid measurements in human plasma and serum. Am. J. Clin. Nutr. 54, 712–716 (1991).
Deng, C., Wynshaw-Boris, A., Zhou, F., Kuo, A. & Leder, P. Fibroblast growth factor receptor 3 is a negative regulator of bone growth. Cell 84, 911–921 (1996).
Acknowledgements
This study was supported by the Divisions of Intramural Research of the National Human Genome Research Institute and the National Institute of Diabetes and Digestive and Kidney Diseases and the Veterinary Resources Program of the National Institutes of Health.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Sotiriou, S., Gispert, S., Cheng, J. et al. Ascorbic-acid transporter Slc23a1 is essential for vitamin C transport into the brain and for perinatal survival. Nat Med 8, 514–517 (2002). https://doi.org/10.1038/0502-514
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/0502-514
This article is cited by
-
Structural basis of vitamin C recognition and transport by mammalian SVCT1 transporter
Nature Communications (2023)
-
Vitamin C: historical perspectives and heart failure
Heart Failure Reviews (2021)
-
Enteropathogenic Escherichia coli Infection Inhibits Intestinal Ascorbic Acid Uptake via Dysregulation of Its Transporter Expression
Digestive Diseases and Sciences (2021)
-
Identification of placental nutrient transporters associated with intrauterine growth restriction and pre-eclampsia
BMC Genomics (2018)
-
The role of brain barriers in fluid movement in the CNS: is there a ‘glymphatic’ system?
Acta Neuropathologica (2018)