Abstract
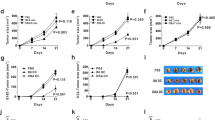
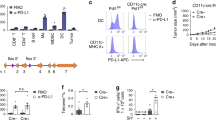
Cytotoxic T lymphocytes and natural killer cells are essential effectors of anti-tumor immune responses in vivo. Dendritic cells (DC) 'prime' tumor antigen-specific cytotoxic T lymphocytes; thus, we investigated whether DC might also trigger the innate, NK cell-mediated anti-tumor immunity. In mice with MHC class I-negative tumors, adoptively transferred- or Flt3 ligand-expanded DC promoted NK cell-dependent anti-tumor effects. In vitro studies demonstrated a cell-to-cell contact between DC and resting NK cells that resulted in a substantial increase in both NK cell cytolytic activity and IFN-γ production. Thus, DC are involved in the interaction between innate and adaptive immune responses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Inaba, K., Metlay, J.P., Crowley, M.T. & Steinman, R.M. Dendritic cells pulsed with protein antigens in vitro can prime antigen-specific, major histocompatibility complex-restricted T cells in situ. J. Exp. Med. 172, 631–640 ( 1990).
Grabbe, S. et al. Tumor antigen presentation by murine epidermal cell. J. Immunol. 146, 3656–3661 (1991).
Zitvogel, L. et al. Therapy of murine tumors with tumor peptide pulsed dendritic cells: dependence on T cells, B7 costimulation, and Th1-associated cytokines. J. Exp. Med. 183, 87–97 (1996).
Celluzzi, C.M., Mayordomo, J.I., Storkus, W.J., Lotze, M.T. & Falo Jr, L.D. Peptide-pulsed dendritic cells induce antigen-specific, CTL-mediated protective tumor immunity. J. Exp. Med. 183, 283–287 (1996).
Banchereau, J. & Steinman, R.M. Dendritic cells and the control of immunity. Nature 392, 245– 252 (1998).
Cui, J. et al. Requirement for Vα14 NKT cells in IL-12-mediated rejection of tumors. Science 278, 1623– 1626 (1997).
Kawano, T. et al. CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science 278, 1626–1629 (1997).
Talmadge, J.E., Meyers, K.M., Prieur, D.J. & Starkey, J.R. Role of natural killer cells in tumor growth and metastasis: C57BL/6 normal and beige mice. Nature 284, 622– 625 (1980).
Trinchieri, G. Biology of natural killer cells. Adv. Immunol. 47, 187–303 (1989).
Lanier, L.L. Follow the leader: NK cell receptors for classical and nonclassical MHC class I. Cell 92, 705–707 (1998).
Kärre, K., Ljunggren, H.G., Piontek, G. & Kiessling, R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defense strategy. Nature 319, 675 –678 (1986).
Salazar-Mather, T.P., Ishikawa, R. & Biron, C.A. NK cell trafficking and cytokine expression in splenic compartments after IFN induction and viral infection. J. Immunol. 157, 3054–3064 ( 1996).
Reis e Sousa, C. et al. In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J. Exp. Med. 186, 1819– 1829 (1997).
Maraskovsky, E. et al. Dramatic increase in the number of functionally mature dendritic cells in Flt3 ligand-treated mice : multiple dendritic cell subpopulations identified. J. Exp. Med. 184, 1953– 1962 (1996).
Shaw, S.G., Maung, A.A., Steptoe, R.J., Thomson, A.W. & Vujanovic, N.L. Expansion of functional natural killer cells in multiple tissue compartments of mice treated with Flt3-ligand: implications for anti-cancer and anti-viral therapy. J. Immunol. 161, 2817–2824 ( 1998).
Moalli, P.A., MacDonald, J.L., Goodglick, L.A. & Kane, A.B. Acute injury and regeneration of the mesothelium in response to asbestos fibers. Am. J. Pathol. 128, 426– 445 (1987).
D'Andrea, A. et al. Production of natural killer cell stimulatory factor (NKSF/IL-12) by peripheral blood mononuclear cells. J. Exp. Med. 176, 1387–1398 (1992).
Ardavin, C., Wu, L., Li, C.-L. & Shortman, K. Thymic dendritic cells and T cells develop simultaneously in the thymus from a common precursor population. Nature 362, 761– 763 (1993).
Winzler, C. et al. Maturation stages of mouse dendritic cells in growth factor-dependent long-term cultures. J. Exp. Med. 185, 317 –328 (1997).
Hart, D.N.J. Dendritic cells: unique leukocyte populations which control the primary immune response. Blood 90, 3245– 3287 (1997).
Inaba, K. et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 176, 1693– 1702 (1992).
Chang, Z.L., Whiteside, T.L. & Herberman, R.B. Immunoregulatory role of in vitro differentiated macrophages on human natural killer (NK)-cell activity. Cell. Immunol. 125, 183–196 (1990).
Montel, A.H., Morse, P.A. & Brahmi, Z. Upregulation of B7 molecules by Epstein-Barr virus enhances susceptibility to lysis by a human NK-like cell line. Cell. Immunol. 160, 101–114 ( 1995).
Carbone, E. et al. A new mechanism of NK cell cytotoxicity activation: the CD40-CD40 ligand interaction. J. Exp. Med. 185, 2053 –2060 (1997).
Chambers, B.J., Salcedo, M. & Ljunggren, H.-G. Triggering of natural killer cells by the costimulatory molecule CD80 (B7-1). Immunity 5, 311– 317 (1996).
Lang, S., Vujanovic, N.L., Wollenberg, B. & Whiteside, T.L. Absence of B7.1-CD28/CTLA-4-mediated co-stimulation in human NK cells. Eur. J. Immunol. 28, 780–786 (1998).
Johnson, L.L. & Sayles, P.C. Interleukin-12, dendritic cells, and the initiation of host-protective mechanisms against Toxoplasma gondii . J. Exp. Med. 186, 1799– 1802 (1997).
Bandyopadhyay, S., Perussia, S., Trinchieri, G., Miller, D.S. & Starr, S.E. Requirement for HLA-DR+ accessory cells in natural killing of cytomegalovirus-infected fibroblasts. J. Exp. Med. 164, 180–195 (1986).
de Saint-Vis, B. et al. The cytokine profile expressed by human dendritic cells is dependent on cell subtype and mode of activation. J. Immunol. 160, 1666–1676 (1998).
Chen, K. et al. Antitumor activity and immunotherapeutic properties of Flt3-ligand in a murine breast cancer model. Cancer Res. 57, 3511–3516 (1997).
Lynch, D.H. et al. Flt3 ligand induces tumor regression and anti-tumor immune responses in vivo. Nature Med. 3, 625– 631 (1997).
Lyman, S.D. & Jacobsen, S.E.W. c-kit ligand and Flt3 ligand: stem/progenitor cell factors with overlapping yet distinct activities. Blood 91, 1101–1107 ( 1998).
Gismondi, A. et al. Human natural killer cells express VLA-4 and VLA-5, which mediate their adhesion to fibronectin. J. Immunol. 146, 384–392 (1991).
Allavena, P. et al. Induction of natural killer cell migration by monocyte chemotactic protein-1, -2 and -3. Eur. J. Immunol. 24, 3233–3236 (1994).
Medzhitov, R. & Janeway Jr, C.A. Innate immunity: impact on the adaptive immune response. Curr. Opin. Immunol. 9, 4–9 (1997).
Chen, J. RAG-2-deficient blastocyst complementation: an assay of gene function in lymphocyte development. Proc. Natl. Acad. Sci. USA 90, 4528–4532 (1993).
Müller, U. et al. Functional role of type I and type II interferons in antiviral defense. Science 264, 1918– 1921 (1994).
Cobbold, S.P., Jayasuriya, A., Nash, A., Prospero, T.D. & Waldmann, H. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature 312, 548–551 (1984).
Wysocka, M. et al. Interleukin-12 is required for interferon-γ production and lethality in lipopolysaccharide-induced shock in mice. Eur. J. Immunol. 25, 672–676 (1995).
Zitvogel, L. et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nature Med. 4, 594–600 (1998).
Acknowledgements
We are grateful to the staff of the Animal Facility of IGR. We thank C. Maliszewski, C. Bonnerot, S. Amigorena, F. Faure and E. Vivier for critical review of the manuscript. We are also indebted to M. Rescigno for contribution with the R1-conditioned medium and to P. Bousso for his input in flow cytometry analyses. E. Mottez is acknowledged for the generation of the P815-B7.1 cell line. This work was supported by the Association pour la Recherche sur le Cancer, the Ligue Nationale de Lutte contre le Cancer, the GEFLUC association, programme "Immunité Antitumorale", the Institut Gustave Roussy, CRC IGR number 97.1, and by CNRS. NF was supported by the Institut de Formation Supérieure Biomédicale, the Fondation pour la Recherche Médicale and Rhône Poulenc RORER. AL was supported by CRC IGR number 97.1.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fernandez, N., Lozier, A., Flament, C. et al. Dendritic cells directly trigger NK cell functions: Cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med 5, 405–411 (1999). https://doi.org/10.1038/7403
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/7403
This article is cited by
-
NK cells are never alone: crosstalk and communication in tumour microenvironments
Molecular Cancer (2023)
-
The NK cell–cancer cycle: advances and new challenges in NK cell–based immunotherapies
Nature Immunology (2020)
-
Sesamolin promotes cytolysis and migration activity of natural killer cells via dendritic cells
Archives of Pharmacal Research (2020)
-
Increased Granulopoiesis in the Bone Marrow following Epstein-Barr Virus Infection
Scientific Reports (2019)
-
Bacterial coinfection restrains antiviral CD8 T-cell response via LPS-induced inhibitory NK cells
Nature Communications (2018)