Abstract
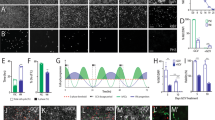
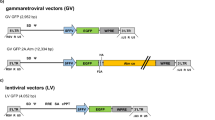
In humans, β–hexosaminidase α–subunit deficiency prevents the formation of a functional β–hexosaminidase A heterodimer resulting in the severe neurodegenerative disorder, Tay–Sachs disease. To explore the feasibility of using ex vivo gene transfer in this lysosomal storage disease, we produced ecotropic retroviruses encoding the human β–hexosaminidase α–subunit cDNA and transduced multipotent neural cell lines. Transduced progenitors stably expressed and secreted high levels of biologically active β–hexosaminidase A in vitro and cross–corrected the metabolic defect in a human Tay–Sachs fibroblasts cell line in vitro. These genetically engineered CMS progenitors were transplanted into the brains of both normal fetal and newborn mice. Engrafted brains, analyzed at various ages after transplant, produced substantial amounts of human β–hexosaminidase α–subunit transcript and protein, which was enzymatically active throughout the brain at a level reported to be therapeutic in Tay–Sachs disease. These results have implications for treating neurologic diseases characterized by inherited single gene mutations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Sandhoff, K., Conzelmann, E., Neufeld, E.F., Kaback, M.M. & Suzuki, K. The GM2 gangliosidosis. in The Metabolic Basis of Inherited Disease 6th edn. (eds. Schriver, C.R., Beaudet, A.L., Sly, W.S. & Valle, D.) 1807–1839 (McGraw Hill, New York, 1989).
Gieselmann, V. Lysosomal storage diseases. Biochim. Biophys. Acta 1270, 103–136 (1995).
Suhr, S.T. & Gage, F.H. Gene therapy for neurologic disease. Arch. Neurol. 50, 1252–1268 (1993).
Sabaté, O. et al. Transplantation to the rat brain of human neural progenitors that were genetically modified using adenoviruses. Nature Genet. 9, 256–260 (1995).
Snyder, E.Y., Taylor, R.M. & Wolfe, J.H. Neural progenitor cell engraftment corrects lysosomal storage throughout the MPS VII mouse brain. Nature 374, 367–370 (1995).
Onifer, S.M., Whittemore, S.R. & Holets, V.R. Variable morphological differentiation of a raphe-derived neuronal cell line following transplantation into the adult rat CNS. Exp. Neurol. 122, 130–142 (1993).
Anton, R. et al. Neural-targeted gene therapy for rodent and primate hemiparkinsonism. Exp. Neurol. 127, 207–218 (1994).
Groves, A.K. et al. Repair of demyelinated lesions by transplantation of purified O-2A progenitor cells. Nature 362, 453–455 (1993).
Renfraz, P.J., Cunningham, M.G. & McKay, R.D.G. Region-specific differentiation of the hippocampal stem cell line HiB5 upon implantation into the developing mammalian brain. Cell 66, 713–719 (1991).
Snyder, E.Y. et al. Multipotent neural cell lines can engraft and participate in development of mouse cerebellum. Cell 68, 33–51 (1992).
Morgenstern, J.P. & Land, H. Advanced mammalian gene transfer: High titer retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 18, 3587–3596 (1990).
Ioannou, Y.A., Bishop, D.F. & Desnick, R.J. Overexpression of human α-galactosidase A results in its intracellular aggregation, crystallization in lysosomes and selective secretion. J. Cell Biol. 119, 1137–1150 (1992).
Braun, S.E. et al. Metabolic correction and cross-correction of mucopolysaccharidosis type II (Hunter syndrome) by retroviral-mediated gene transfer and expression of human iduronate-2-sulfatase. Proc. Natl. Acad. Sci. USA 90, 11830–11834 (1993).
Hasilik, A. & Neufeld, E.F. Biosynthesis of lysosomal enzymes in fibroblasts: Synthesis as precursor of higher molecular weight. J. Biol. Chem. 255, 4937–4945 (1980).
Proia, R.L., d'Azzo, A. & Neufeld, E.F. Association of alpha- and beta-subunits during the biosynthesis of beta-hexosaminidase in cultured human fibroblasts. J. Biol Chem. 259, 3350–3354 (1984).
Lacorazza, H.D. & Jendoubi, M. In situ assessment of β-hexosaminidase activity. BioTechniques 19, 434–439 (1995).
Kresse, H., Fuchs, W., Glossl, J., Holtfrerich, D. & Gilberg, W. Liberation of N-acetylglucosamine-6-sulfate by human β-N-acetylhexosaminidase. A. J. Biol. Chem. 256, 12926–12932 (1981).
Yamanaka, S., Johnson, O.N., Norflus, F., Boles, D.J. & Proia, R.L. Structure and expression of the mouse β-hexosaminidase genes, Hexa and Hexb. Genomics 21, 588–596 (1994).
Wileman, T., Harding, C. & Stahl, P. Receptor-mediated endocytosis. Biochem. J. 232, 1–14 (1985).
Nolan, C.M. & Sly, W.S. I-cell disease and pseudo-Hurler polydystrophy: Disorders of lysosomal enzyme phosphorylation and localization. in The Metabolic Basis of Inherited Disease, 6th edn. (eds. Schriver, C.R., Beaudet, A.L., Sly, W.S. & Valle, D.) 1589–1601 (McGraw Hill, New York, 1989).
Leinekugel, P., Michel, S., Conzelmann, E. & Sandhoff, K. Quantitative correlation between the residual activity of β-hexosaminidase A and arylsulfatase A and the severity of the resulting lysosomal storage disease. Hum. Genet. 88, 513–523 (1992).
Yamanaka, S. et al. Targeted disruption of the Hexa gene results in mice with biochemical and pathologic features of Tay-Sachs disease. Proc. Natl. Acad. Sci. USA 91, 9975–9979 (1994).
Chen, A.C. & Okayama, H. Calcium phosphate-mediated gene transfer: A highly efficient transfection system for stably transforming cells with plasmid DNA. BioTechniques 6, 632–638 (1988).
Chirgwin, J.M., Przybyla, A.E., MacDonald, R.J. & Rutter, W.J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry 18, 5294–5299 (1979).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lacorazza, H., Flax, J., Snyder, E. et al. Expression of human β–hexosaminidase α–subunit gene (the gene defect of Tay–Sachs disease) in mouse brains upon engraftment of transduced progenitor cells. Nat Med 2, 424–429 (1996). https://doi.org/10.1038/nm0496-424
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nm0496-424
This article is cited by
-
Sphingolipid lysosomal storage diseases: from bench to bedside
Lipids in Health and Disease (2021)
-
Upregulating β-hexosaminidase activity in rodents prevents α-synuclein lipid associations and protects dopaminergic neurons from α-synuclein-mediated neurotoxicity
Acta Neuropathologica Communications (2020)
-
Gene Therapy for the Nervous System: Challenges and New Strategies
Neurotherapeutics (2014)
-
Clinical translation of human neural stem cells
Stem Cell Research & Therapy (2013)
-
Gene Transfer Corrects Acute GM2 Gangliosidosis—Potential Therapeutic Contribution of Perivascular Enzyme Flow
Molecular Therapy (2012)