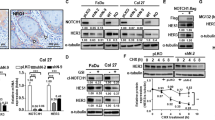
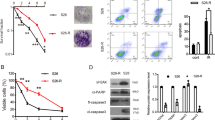
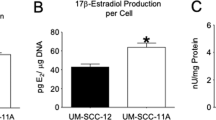
Abstract
Retinoic acid (RA) has been shown to be effective in eradicating premalignant lesions1 and preventing second primary malignancies in patients cured of squamous cell carcinoma of the head and neck (SCCHN) in clinical trials2. The basis for this effect is unclear. We have previously demonstrated that messenger RNA from tumor growth factor–α (TGF–α) and its receptor, the epidermal growth factor (EGFR), is upregulated in tumors and histologically normal mucosal samples from patients with SCCHN compared with control normal mucosa from patients without cancer, implicating this ligand–receptor pair in an autocrine growth pathway early in the molecular pathogenesis of this disease3. In this report, we examined the hypothesis that the action of RA on the mucosa of the upper aerodigestive tract is mediated via downregulation of steady–state TGF–α and/or EGFR mRNA levels. Following exposure to all–trans–RA, a series of SCCHN cell lines demonstrated a 35.4% reduction in TGF–α mRNA expression (P= 0.022) and 58.5% reduction in EGFR mRNA (P = 0.0027). Nuclear run–on analysis indicated that the RA–mediated reduction of TGF–α and EGFR steady–state mRNA levels was a result of decreased gene transcription. These results suggest that the clinical effects of RA in SCCHN patients may be due to a downmodulation of TGF–α and EGFR mRNA production.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Lotan, R. et al. Suppression of retinoic acid receptor-β in premalignant oral lesions and its up-regulation by isotretinoin. N. Engl. J. Med. 332, 1405–1410 (1995).
Hong, W.K. et al. Prevention of second primary tumors with isotretinoin in squamous cell carcinoma of the head and neck. N. Engl. J. Med. 323, 795–801 (1990).
Rubin Grandis, J. & Tweardy, D.T. Elevated levels of transforming growth factor-α and epidermal growth factor receptor messenger RNA are early markers of carcinogenesis in head and neck cancer. Cancer Res. 53, 3579–3584 (1993).
Field, J.K. et al. Elevated expression of the c-myc oncoprotein correlates with poor prognosis in head and neck squamous cell carcinoma. Oncogene 4, 1463–1468 (1989).
Boyle, J.O. et al. The incidence of p53 mutations increases with progression of head and neck cancer. Cancer Res. 53, 4477–4480 (1993).
Todd, R. et al. TGF-α and EGF receptor mRNA in human oral cancers. Carcinogenesis 10, 1553–1556 (1989).
Williams, M.E. et al. Chromosome 11ql3 amplification in head and neck squamous cell carcinoma. Arch. Otolaryngol. Head Neck Surg. 119, 1238–1243 (1993).
Maestro, R. et al. Three discrete regions of deletion at 3p in head and neck cancers. Cancer Res. 53, 5775–5779 (1993).
Nawroz, H. et al. Allelotype of head and neck squamous cell carcinoma. Cancer Res. 54, 1152–1155 (1994).
Schuuring, E., Verhoeven, E., Mooi, W.J. & Michalides, R.J. Identification and cloning of two overexpressed genes U21B31/PRD 1 and EMS 1, within the amplified chromosome 11ql3 region in human carcinomas. Oncogene 7, 355–361 (1992).
Callender, T. et al. PRAD-1 (CCNDl)/Cyclin Dl oncogene amplification in primary head and neck squamous cell carcinomas. Cancer 74, 152–158 (1994).
Warrell, R.P., Jr., de The, H., Wang, Z.Y. & Dego, S.L. Acute promyelocytic leukemia. N. Engl. J. Med. 329, 177–189 (1993).
Mangelsdorf, D.J., Ong, E.S., Dyck, J.A. & Evans, R.M. Nuclear receptor that identifies a novel retinoic acid response pathway. Nature 345, 224–229 (1990).
Stellmach, V., Leask, A. & Fuchs, E. Retinoid-mediated transcriptional regulation of keratin genes in human epidermal and squamous cell carcinoma cells. Proc. Natl. Acad. Sci. USA 88, 4582–4586 (1991).
Kim, J.S. et al. Suppression by retinoic acid of epidermal growth factor receptor autophosphorylation and glycosylation in cultured human head and neck squamous carcinoma cells. J. Natl. Cancer Inst. Monogr. 13, 101–110 (1992).
Zheng, Z.S. & Goldsmith, L.A. Modulation of epidermal growth factor receptors by retinoic acid in ME180 cells. Cancer Res. 50, 1201–1205 (1990).
Jetten, A.M. et al. Inhibition of growth and squamous cell differentiation markers in cultured human head and neck squamous carcinoma cells by β-all-trans retinoic acid. Int. J. Cancer 45, 195–202 (1990).
Sacks, P.G., Oke, V., Amos, B., Vasey, T. & Lotan, R. Modulation of growth, differentiation and glycoprotein synthesis by β-all-trans retinoic acid in a multicellular tumor spheroid model for squamous carcinoma of the head and neck. Int. J. Cancer 44, 926–933 (1989).
Dmitrovsky, E., Moy, D., Miller, W.H., Li, A. & Masui, H. Retinoic acid causes a decline in TGF-α expression, cloning efficiency, and tumorigenicity in a human embryonal cancer cell line. Oncogene Res 5, 233–239 (1990).
Garewal, H.S. et al. Response of oral leukoplakia to β-carotene. J. Clin. Oncol. 8, 1715–1720 (1990).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Grandis, J., Zeng, Q. & Tweardy, D. Retinoic acid normalizes the increased gene transcription rate of TGF–α and EGFR in head and neck cancer cell lines. Nat Med 2, 237–240 (1996). https://doi.org/10.1038/nm0296-237
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nm0296-237
This article is cited by
-
Predictive value of EGFR and HER2 overexpression in advanced non-small-cell lung cancer
Oncogene (2009)
-
FcγRIIIa polymorphisms and cetuximab induced cytotoxicity in squamous cell carcinoma of the head and neck
Cancer Immunology, Immunotherapy (2009)
-
Role of epidermal growth factor receptor degradation in gemcitabine-mediated cytotoxicity
Oncogene (2007)
-
Combination of radiotherapy with EGFR antagonists for head and neck carcinoma
International Journal of Clinical Oncology (2007)
-
Induction of cyclooxygenase-2 by benzo[a]pyrene diol epoxide through inhibition of retinoic acid receptor-β2 expression
Oncogene (2005)