Abstract
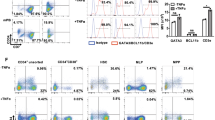
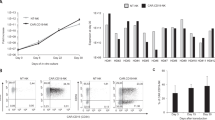
Analysis of the events that regulate development of red blood cells or granulocytes has led to therapies altering clinical conditions associated with anemia or neutropenia. The development of therapeutic approaches to target conditions associated with lymphopenia, such as AIDS, has been thwarted by limited techniques for studying T–lymphocyte development. We describe an in vitro system in which human bone marrow CD34+ cells proliferate, acquire the expression of the lymphoid–specific RAG–2 gene and a broad repertoire of rearranged T–cell receptor genes, develop the ability to produce T cell–specific interleukin–2 and achieve a range of T–cell immunophenotypes. The cells also become susceptible to infection with the T–lymphotropic strain of human immunodeficiency virus–1, HIV–1IIIB. This culture system induces human T lymphopoiesis and may permit further analysis of the events regulating human T–lineage differentiation. It provides a preclinical model for screening stem cell gene therapies directed toward AIDS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Dexter, T.M., Allen, T.D. & Lajha, L.G. Conditions controlling the proliferation of hemopoietic stem cells in vitro. J. Cell. Physiol. 91, 335–344 (1977).
Whitlock, C.A. & Witte, O.N. Long-term culture of B lymphocytes and their precursors from murine bone marrow. Proc. Natl. Acad. Sci. USA 79, 3608–3612 (1982).
Haynes, B., Martin, M., Kay, H. & Kurtzberg, J. Early events in human T cell ontogeny. J. Exp. Med. 168, 1061–1080 (1988).
Kurtzberg, J., Denning, S., Nycum, L., Singer, K. & Haynes, B. Immature human thymocytes can be driven to differentiate into nonlymphoid lineages by cy-tokines from thymic epithelial cells. Proc. Natl. Acad. Sci. USA 86, 7575–7579 (1989).
Jenkinson, E.J., Franchi, L.L., Kingston, R. & Owen, J.J.T. Effect of deoxyguanosine on lymphopoiesis in the developing thymus rudiment in vitro: Application in the production of chimeric thymus rudiments. Eur. J. Immunol. 12, 583–587 (1982).
Yeoman, H. et al. Human bone marrow and umbilical cord blood cells generate CD4+ and CD8+ single-positive T cells in murine fetal thymus organ culture. Immunology 90, 10778–10782 (1993).
Galy, A., Verma, S., Barcena, A. & Spits, H. Precursors of CD3+CD4+CD8+ cells in the human thymus are defined by expression of CD34: Delineation of early events in human thymic development. J. Exp. Med. 178, 391–401 (1993).
Peault, B., Weissman, I.L., Baum, C., McCune, J.M. & Tsukamoto, A. Lymphoid reconstitution of the human fetal thymus in SCID mice with CD34+ precursor cells. J. Exp. Med. 174, 1283–1286 (1991).
Akkina, R.K., Rosenblatt, J.D., Campbell, A.G., Chen, I.S.Y. & Zack, J.A. Modeling human lymphoid precursor cell gene therapy in the SCID-hu mouse. Blood 84, 1393–1398 (1994).
Lyerly, H.K., Matthews, T.J., Langlois, A.J., Bolognesi, D.P. & Winhold, K.J., T-cell lymphotropic virus IIIB glycoprotein (gp120) bound to CD4 determinants on normal lymphocytes and expressed by infected cells serves as target for immune attack. Proc. Natl. Acad. Sci. USA 84, 4601–4605 (1987).
Aldrovandi, G.M. et al. The SCID-hu mouse as a model for HIV-1 infection. Nature 363, 732–736 (1993).
Bonyhadi, M.L. et al. HIV induces thymus depletion in vivo. Nature 363, 728–732 (1993).
Su, L. et al. HIV-1 induced thymocyte depletion is associated with indirect cytopathicity and infection of progenitor cells in vivo. Immunity 2, 25–36 (1995).
Stanley, S.K. et al. Human immunodeficiency virus infection of the human thymus and disruption of the thymic microenvironment in the SCID-hu mouse. J. Exp. Med. 178, 1151–1163 (1993).
Tanaka, K.E. et al. HIV-1 infection of human fetal thymocytes. J. Acauir. Immune Deftc. Syndr. 5, 94–101 (1992).
Rosenzweig, M., Clark, D.P. & Gaulton, G.N. Selective thymocyte depletion in neonatal HIV-1 thymic infection. AIDS 7, 1601–1605 (1993).
Oettinger, M.A., Schatz, D.G., Gorka, C. & Baltimore, D. RAG-1 and RAG-2, adjacent genes that synergistically activate V(D)J recombination. Science 248, 1517–1523 (1990).
Kalams, S.A. et al. Longitudinal analysis of T cell receptor (TCR) gene usage by human immunodeficiency virus 1 envelope-specific cytotoxic T lymphocyte clones reveals a limited TCR repertoire. J. Exp. Med. 179, 1261–1271 (1994).
Wolf, S.F., Sieburth, D. & Sypek, J. Interleukin 12: A key modulator of immune function. Stem Cells 12, 154–168 (1994).
Jacobsen, S.E.W., Veiby, O.P. & Smeland, E.B. Cytotoxic lymphocyte maturation factor (interleukin 12) is a synergistic growth factor for hematopoietic stem cells. J. Exp. Med. 178, 413–418 (1993).
Hirayama, F. et al. Synergistic interaction between interleukin-12 and steel factor in support of proliferation of murine lymphohematopoietic progenitors in culture. Blood 83, 92–98 (1994).
Godfrey, D.I. et al. IL-12 influences intrathymic T cell development. J. Immunol. 152, 2729–2734 (1994).
Kobayashi, M. et al. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biological effects on human lymphocytes. J. Exp. Med. 170, 827–845 (1989).
Lyman, S.D. et al. Molecular cloning of a ligand for the flt3/flk-2 tyrosine ki-nase receptor: A proliferative factor for primitive hematopoietic cells. Cell 75, 1157–1167 (1993).
Hannum, C. et al. Ligand for FLT3/FLK2 receptor tyrosine kinase regulates growth of haematopoietic stem cells and is encoded by variant RNAs. Nature 368, 643–648 (1994).
Jacobsen, S.E.W., Okkenhaug, C., Myklebust, J., Veilby, O.P. & Lyman, S.D. The FLT3 ligand potently and directly stimulates the growth and expansion of primitive murine bone marrow progenitor cells in vitro: Synergistic interactions with interleukin (IL) 11, IL-12, and other hematopoietic growth factors. J. Exp. Med. 181, 1357–1363 (1995).
Terstappen, L.W.W.N., Huang, S. & Picker, L.J. Flow cytometric assessment of human T-cell differentiation in thymus and bone marrow. Blood 79, 666–677 (1992).
Kraft, D.L., Weissman, I.L. & Waller, E.K. Differentiation of CD3–4–8– human fetal thymocytes in vivo: Characterization of CD3–4+8– intermediate. J. Exp. Med. 178, 265–277 (1993).
Tjonnfjord, G.E., Veilby, O.P., Steen, R. & Egeland, T. T lymphocyte differentiation in vitro from adult human prethymic CD34+ bone marrow cells. J. Exp. Med. 177, 1531–1539 (1993).
Ikuta, K. et al. A developmental switch in thymic lymphocyte maturation potential occurs at the level of hematopoietic stem cells. Cell 62, 863–874 (1990).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Freedman, A., Zhu, H., Levine, J. et al. Generation of human T lymphocytes from bone marrow CD34+ cells in vitro. Nat Med 2, 46–51 (1996). https://doi.org/10.1038/nm0196-46
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nm0196-46
This article is cited by
-
Mesenchymal stromal cells support the viability and differentiation of thymocytes through direct contact in autologous co-cultures
Histochemistry and Cell Biology (2016)
-
Modulation and the Underlying Mechanism of T Cells in Thymus of Mice by Oral Administration of Sodium Fluoride
Biological Trace Element Research (2016)
-
Extrathymic T-cell differentiation in vitro
Nature Reviews Immunology (2004)
-
Amplification of T cells from human cord blood in serum-deprived culture stimulated with stem cell factor, interleukin-7 and interleukin-2
Bone Marrow Transplantation (2003)
-
Genetically modified immunocompetent cells in HIV infection
Gene Therapy (2001)