Abstract
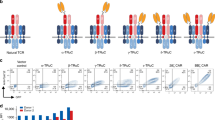
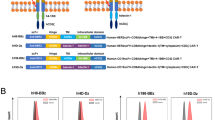
The adoptive transfer of T cells engineered with a chimeric antigen receptor (CAR) (hereafter referred to as CAR-T cells) specific for the B lymphocyte antigen CD19 has shown impressive clinical responses in patients with refractory B cell malignancies1,2,3,4,5,6,7. However, the therapeutic effects of CAR-T cells that target other malignancies have not yet resulted in significant clinical benefit8,9,10,11. Although inefficient tumor trafficking and various immunosuppressive mechanisms can impede CAR-T cell effector responses, the signals delivered by the current CAR constructs may still be insufficient to fully activate antitumor T cell functions. Optimal T cell activation and proliferation requires multiple signals, including T cell receptor (TCR) engagement (signal 1), co-stimulation (signal 2) and cytokine engagement (signal 3)12. However, CAR constructs currently being tested in the clinic contain a CD3z (TCR signaling) domain and co-stimulatory domain(s) but not a domain that transmits signal 3 (refs. 13, 14, 15, 16, 17, 18). Here we have developed a novel CAR construct capable of inducing cytokine signaling after antigen stimulation. This new-generation CD19 CAR encodes a truncated cytoplasmic domain from the interleukin (IL)-2 receptor β-chain (IL-2Rβ) and a STAT3-binding tyrosine-X-X-glutamine (YXXQ) motif, together with the TCR signaling (CD3z) and co-stimulatory (CD28) domains (hereafter referred to as 28-ΔIL2RB-z(YXXQ)). The 28-ΔIL2RB-z(YXXQ) CAR-T cells showed antigen-dependent activation of the JAK kinase and of the STAT3 and STAT5 transcription factors signaling pathways, which promoted their proliferation and prevented terminal differentiation in vitro. The 28-ΔIL2RB-z(YXXQ) CAR-T cells demonstrated superior in vivo persistence and antitumor effects in models of liquid and solid tumors as compared with CAR-T cells expressing a CD28 or 4-1BB co-stimulatory domain alone. Taken together, these results suggest that our new-generation CAR has the potential to demonstrate superior antitumor effects with minimal toxicity in the clinic and that clinical translation of this novel CAR is warranted.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Brentjens, R.J. et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci. Transl. Med. 5, 177ra38 (2013).
Grupp, S.A. et al. Chimeric-antigen-receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 368, 1509–1518 (2013).
Maude, S.L. et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 371, 1507–1517 (2014).
Lee, D.W. et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukemia in children and young adults: a phase 1 dose-escalation trial. Lancet 385, 517–528 (2015).
Davila, M.L. et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl. Med. 6, 224ra25 (2014).
Porter, D.L. et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci. Transl. Med. 7, 303ra139 (2015).
Kochenderfer, J.N. et al. B cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 119, 2709–2720 (2012).
Till, B.G. et al. CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4-1BB domains: pilot clinical trial results. Blood 119, 3940–3950 (2012).
Ahmed, N. et al. Human epidermal growth factor receptor 2 (HER2)-specific chimeric antigen receptor-modified T cells for the immunotherapy of HER2-positive sarcoma. J. Clin. Oncol. 33, 1688–1696 (2015).
Kershaw, M.H. et al. A phase 1 study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin. Cancer Res. 12, 6106–6115 (2006).
Pule, M.A. et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat. Med. 14, 1264–1270 (2008).
Kershaw, M.H., Westwood, J.A. & Darcy, P.K. Gene-engineered T cells for cancer therapy. Nat. Rev. Cancer 13, 525–541 (2013).
Kowolik, C.M. et al. CD28 co-stimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T cells. Cancer Res. 66, 10995–11004 (2006).
Brentjens, R.J. et al. Genetically targeted T cells eradicate systemic acute lymphoblastic leukemia xenografts. Clin. Cancer Res. 13, 5426–5435 (2007).
Stephan, M.T. et al. T cell–encoded CD80 and 4-1BBL induce auto- and trans-co-stimulation, resulting in potent tumor rejection. Nat. Med. 13, 1440–1449 (2007).
Milone, M.C. et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol. Ther. 17, 1453–1464 (2009).
Savoldo, B. et al. CD28 co-stimulation improves expansion and persistence of chimeric-antigen-receptor-modified T cells in lymphoma patients. J. Clin. Invest. 121, 1822–1826 (2011).
Lim, W.A. & June, C.H. The principles of engineering immune cells to treat cancer. Cell 168, 724–740 (2017).
Rochman, Y., Spolski, R. & Leonard, W.J. New insights into the regulation of T cells by γc family cytokines. Nat. Rev. Immunol. 9, 480–490 (2009).
Zeng, R. et al. The molecular basis of IL-21-mediated proliferation. Blood 109, 4135–4142 (2007).
Hinrichs, C.S. et al. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood 111, 5326–5333 (2008).
Xin, G. et al. A critical role of IL-21-induced BATF in sustaining CD8 T cell–mediated chronic viral control. Cell Rep. 13, 1118–1124 (2015).
Zeng, R. et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J. Exp. Med. 201, 139–148 (2005).
Markley, J.C. & Sadelain, M. IL-7 and IL-21 are superior to IL-2 and IL-15 in promoting human T cell–mediated rejection of systemic lymphoma in immunodeficient mice. Blood 115, 3508–3519 (2010).
Quintarelli, C. et al. Coexpression of cytokine and suicide genes to enhance the activity and safety of tumor-specific cytotoxic T lymphocytes. Blood 110, 2793–2802 (2007).
Pegram, H.J. et al. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood 119, 4133–4141 (2012).
Hsu, C. et al. Cytokine-independent growth and clonal expansion of a primary human CD8+ T cell clone following retroviral transduction with the IL15 gene. Blood 109, 5168–5177 (2007).
Zhang, L. et al. Tumor-infiltrating lymphocytes genetically engineered with an inducible gene encoding interleukin-12 for the immunotherapy of metastatic melanoma. Clin. Cancer Res. 21, 2278–2288 (2015).
Stahl, N. et al. Choice of STATs and other substrates specified by modular tyrosine-based motifs in cytokine receptors. Science 267, 1349–1353 (1995).
Nicholson, I.C. et al. Construction and characterization of a functional CD19-specific single chain Fv fragment for immunotherapy of B lineage leukemia and lymphoma. Mol. Immunol. 34, 1157–1165 (1997).
Gattinoni, L. et al. A human memory T cell subset with stem-cell-like properties. Nat. Med. 17, 1290–1297 (2011).
Marzec, M. et al. Oncogenic kinase NPM–ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1). Proc. Natl. Acad. Sci. USA 105, 20852–20857 (2008).
Lastwika, K.J. et al. Control of PD-L1 expression by oncogenic activation of the AKT-mTOR pathway in non-small-cell lung cancer. Cancer Res. 76, 227–238 (2016).
Eyquem, J. et al. Targeting a CAR to the TRAC locus with CRISPR–Cas9 enhances tumor rejection. Nature 543, 113–117 (2017).
Cherkassky, L. et al. Human CAR-T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J. Clin. Invest. 126, 3130–3144 (2016).
Sabatino, M. et al. Generation of clinical-grade CD19-specific CAR-modified CD8+ memory stem cells for the treatment of human B cell malignancies. Blood 128, 519–528 (2016).
Cieri, N. et al. IL-7 and IL-15 instruct the generation of human memory stem T cells from naive precursors. Blood 121, 573–584 (2013).
Cui, W., Liu, Y., Weinstein, J.S., Craft, J. & Kaech, S.M. An interleukin-21–interleukin-10–STAT3 pathway is critical for functional maturation of memory CD8+ T cells. Immunity 35, 792–805 (2011).
Siegel, A.M. et al. A critical role for STAT3 transcription factor signaling in the development and maintenance of human T cell memory. Immunity 35, 806–818 (2011).
Durant, L. et al. Diverse targets of the transcription factor STAT3 contribute to T cell pathogenicity and homeostasis. Immunity 32, 605–615 (2010).
Butler, M.O. et al. Ex vivo expansion of human CD8+ T cells using autologous CD4+ T cell help. PLoS One 7, e30229 (2012).
Jedema, I., van der Werff, N.M., Barge, R.M., Willemze, R. & Falkenburg, J.H. New CFSE-based assay to determine susceptibility to lysis by cytotoxic T cells of leukemic precursor cells within a heterogeneous target cell population. Blood 103, 2677–2682 (2004).
Acknowledgements
This work was supported by CIHR Project Grant 362860 (N.H.), Ontario Institute for Cancer Research Clinical Investigator Award IA-039 (N.H.), BioCanRX Catalyst Program grant FY17CAT7 (N.H.), the Princess Margaret Cancer Foundation (M.O.B. and N.H.), a Japan Society for the Promotion of Science Postdoctoral Fellowship for Overseas Researchers (Y.K.), a Guglietti Fellowship Award (Y.K.), a Canadian Institutes of Health Research Canada Graduate Scholarship (T.G.), the Province of Ontario (T.G. and M.A.) and a Natural Sciences and Engineering Research Council of Canada Postgraduate Scholarship (T.G.). This study was partly sponsored by Takara Bio, Inc.
Author information
Authors and Affiliations
Contributions
Y.K. and N.H. designed the project; Y.K., S.T., T.G., M.A., C.-H.W. and K.S. performed the experiments; M.D.M. and M.O.B. provided critical human samples and contributed to the writing of the manuscript; and Y.K. and N.H. analyzed the results and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
S.T. is an employee of Takara Bio, Inc. This study was partly sponsored by Takara Bio, Inc. The University Health Network has filed a patent application related to this study on which N.H., Y.K. and S.T. are named as inventors.
Supplementary information
Supplementary Figures & Tables
Supplementary Figures 1–18 & Supplementary Tables 1–3 (PDF 9605 kb)
Rights and permissions
About this article
Cite this article
Kagoya, Y., Tanaka, S., Guo, T. et al. A novel chimeric antigen receptor containing a JAK–STAT signaling domain mediates superior antitumor effects. Nat Med 24, 352–359 (2018). https://doi.org/10.1038/nm.4478
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.4478
This article is cited by
-
Synthetic biology approaches for improving the specificity and efficacy of cancer immunotherapy
Cellular & Molecular Immunology (2024)
-
Lyophilized lymph nodes for improved delivery of chimeric antigen receptor T cells
Nature Materials (2024)
-
Synthetic receptor scaffolds significantly affect the efficiency of cell fate signals
Scientific Reports (2024)
-
Programmable synthetic receptors: the next-generation of cell and gene therapies
Signal Transduction and Targeted Therapy (2024)
-
Genetic engineering of regulatory T cells for treatment of autoimmune disorders including type 1 diabetes
Diabetologia (2024)